Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help showing my work and solving these problems please!! Solve the following equations: 3. Percent Composition from Mass Problem - When a 13.60g sample
need help showing my work and solving these problems please!! 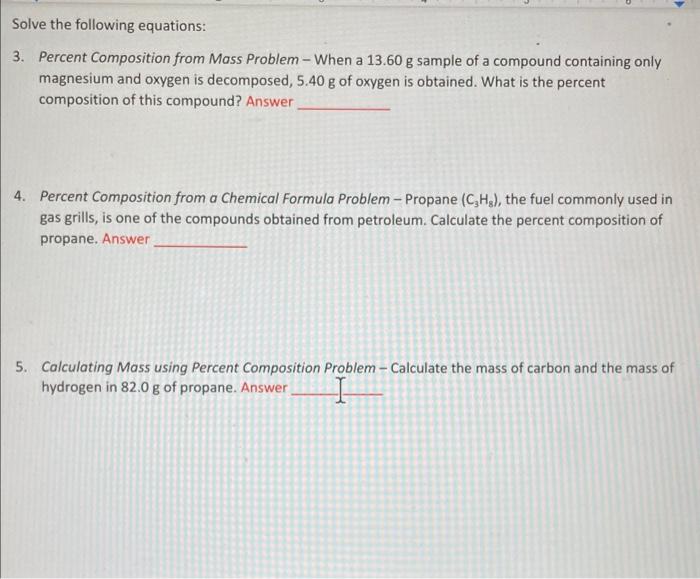
Solve the following equations: 3. Percent Composition from Mass Problem - When a 13.60g sample of a compound containing only magnesium and oxygen is decomposed, 5.40g of oxygen is obtained. What is the percent composition of this compound? Answer 4. Percent Composition from a Chemical Formula Problem - Propane (C3H8), the fuel commonly used in gas grills, is one of the compounds obtained from petroleum. Calculate the percent composition of propane. Answer 5. Calculating Mass using Percent Composition Problem - Calculate the mass of carbon and the mass of hydrogen in 82.0g of propane 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


