Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help with datasheet calculations plz thnak u Unknown #: 1 Unknown Solute Mass of solute is) Mass of water Mass of ice Il Total
need help with datasheet calculations plz
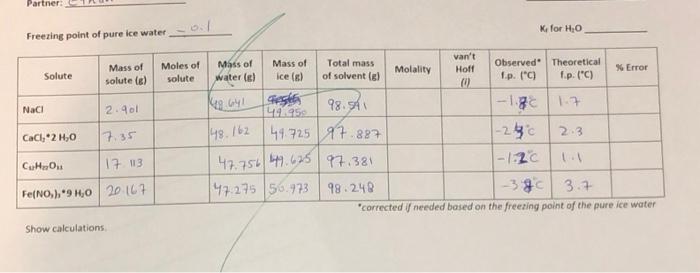
Unknown #: 1 Unknown Solute Mass of solute is) Mass of water Mass of ice Il Total mass of solvent el AT 1 Observed Corrected Calculated Ip. (C Ip (C) MW [/mol) 2. Trial 1 1-220 15.048 15.143 48.661 49.656 98.325 148,754 50.656 99.41 Trial 2 .220 2.1 "needed based on freeing point of pure ice water Average Molecular Weight Identity of Unknown Solute Show calculations Partner K for H.O Freezing point of pure ice water- Solute Mass of solute (6) Moles of solute NaCl 2.901 Ng G4L CaCl, 2 H,0 17.35 148.162 van't Mass of Mass of Total mass Observed Theoretical Molality Holl * Error water(s) ice () of solvent (el I.p. (C) fp. (C) segles 98.5A -1.3 1.7 446959 44725 97.827 1-280 2.3 47.756 49.625 97.381 1-1:20 47.275 50.973 98.248 -380 3.7 'corrected / needed based on the freezing point of the pure ice water CHO 17 13 Fe(NO)940 20 147 Show calculations Possible une Lithium floride (25.9 g/mol) Potassium chloride (74.55 gtmol ) Glycine (75.1 g/ml) Potassium Acethte (98 1 g/mot) ) Mag. Chloride Hexahydrate (203,3 gined) Mag, Iodice (278 gland) Modified freezing Point Depression Experiment Top loading balances will be used for this experiment. Remember, the masses indicated of the solute and solvent do not need to be exact, the actual masses just need to be recorded. Students will use a Vernier temperature probe and department laptop for monitoring temperate change during the experiment. The temperature probe must be rinsed thoroughly with RO water between samples to avoid contamination of solutions. For uniformity in solution temperature, a stir bar is provided for use with a stir plate. Do not operate the stirrer above 300 rpm to avoid splashing of sample and generation of heat. Students will perform this experiment with 4 different known salts as well as 2 trials of an unknown salt. Students will then determine the molar mass of the unknown analyzed using the freezing point data obtained. For successful results, it is essential that all solute be dissolved completely prior to the addition of ice. Continuous swirling or stirring is sufficient, but it may take a few minutes for some of the salts. Waste Handling: Ferric nitrate solutions are collected as hazardous waste - a 4L jug is provided in Waste Hood All other solutions may be flushed down the drain with plenty of water Procedure 1. Fill a 250 ml beaker with about 50 mL of water and crushed ice to make a slurry. Place the beaker with the ice slurry on the stir plate. Select "Collect" on the laptop screen, and immediately lower the temperature probe into the mixture. The program will record a temperature reading every 0.5 seconds for 4 minutes. Use the lowest temperature recorded. a. If the lowest temperature is not 0.0C, a correction will be needed in the calculations to correct for errors in subsequent measurements. 2. Empty the beaker and rinse & dry it thoroughly for future use. There are (3) 250 ml beakers in the lab drawer. Clean the used ones as you go during the 4 minutes of waiting for the temperature recordings. 3. Tare a clean, empty 250 ml beaker. Add and Record the mass of solute required. The known solute masses are specified below. Each unknown is labelled with the mass required as well as the van't Hoff factor. a. NaCl (sodium chloride) - 2.91g b. C12H2O11 (sucrose) - 17.118 c. CaCl 2 H2O (calcium chloride dihydrate) - 7.358 d. Fe(NO))*9 H20 (ferric nitrate nonahydrate) 20.198 4. Tare the beaker + solute. Remove the balance from the beaker and add 50 mL of Ro water to the solute in the beaker Modified Freezing Point Depression Experiment 5. On the tared balance, weigh the beakersolute. water. Record the mass of the 50 ml water. Add the clean stir bar to the solution 5. Stir or swirl the solution until the solute is completely dissolved. You may use the stirplate for this step. All solid must be dissolved before moving on 7. Tare a 100 ml beaker. Add 50 g of ice to the beaker recording the exact mass. 8. Pour the ice into the 250 ml beaker with the solution and immediately lower the temperature probe into the mixture. Press Collect on the laptop screen. Make sure all the ice goes into the beaker and that the temperature probe is not touching the beaker. 9. Swirl ice/solution mixture for 5 seconds to accelerate mixing, then allow to stir undisturbed at 300 rpm for the remainder of the program run time. 10. One the four-minute program ends, verify there is still ice in the mixture. If there is, record the lowest temperature obtained for the mixture. (As long as there is ice remaining the solution is still at its freezing point.) Consult your GTA if there is no ice present. 11. Dispose of the solution as specified in "Waste Handling : Rinse the probe thoroughly with RO water and dry. Dry all glassware thoroughly before using again. 12. Repeat Steps 3 through 11 with each remaining salt and the assigned unknown sample. You will perform this procedure 6 times in total, Calculations Note: Use the carbon copy pages in the back of your manual if you need more room to show your calculations. These will be turned in with your report. Calculate the molality of each of known solution Calculate the theoretical freezing point of each known solution Calculate the error of the observed FP from the theoretical FP calculated Calculate the experimentally determined AT, for each unknown analysis. Use the experimentally determined AT in calculated the molecular weight of the unknown sample Report the average molecular weight determined. Remember: moles = a/MW Unknown #: 1 Unknown Solute Mass of solute is) Mass of water Mass of ice Il Total mass of solvent el AT 1 Observed Corrected Calculated Ip. (C Ip (C) MW [/mol) 2. Trial 1 1-220 15.048 15.143 48.661 49.656 98.325 148,754 50.656 99.41 Trial 2 .220 2.1 "needed based on freeing point of pure ice water Average Molecular Weight Identity of Unknown Solute Show calculations Partner K for H.O Freezing point of pure ice water- Solute Mass of solute (6) Moles of solute NaCl 2.901 Ng G4L CaCl, 2 H,0 17.35 148.162 van't Mass of Mass of Total mass Observed Theoretical Molality Holl * Error water(s) ice () of solvent (el I.p. (C) fp. (C) segles 98.5A -1.3 1.7 446959 44725 97.827 1-280 2.3 47.756 49.625 97.381 1-1:20 47.275 50.973 98.248 -380 3.7 'corrected / needed based on the freezing point of the pure ice water CHO 17 13 Fe(NO)940 20 147 Show calculations Possible une Lithium floride (25.9 g/mol) Potassium chloride (74.55 gtmol ) Glycine (75.1 g/ml) Potassium Acethte (98 1 g/mot) ) Mag. Chloride Hexahydrate (203,3 gined) Mag, Iodice (278 gland) Modified freezing Point Depression Experiment Top loading balances will be used for this experiment. Remember, the masses indicated of the solute and solvent do not need to be exact, the actual masses just need to be recorded. Students will use a Vernier temperature probe and department laptop for monitoring temperate change during the experiment. The temperature probe must be rinsed thoroughly with RO water between samples to avoid contamination of solutions. For uniformity in solution temperature, a stir bar is provided for use with a stir plate. Do not operate the stirrer above 300 rpm to avoid splashing of sample and generation of heat. Students will perform this experiment with 4 different known salts as well as 2 trials of an unknown salt. Students will then determine the molar mass of the unknown analyzed using the freezing point data obtained. For successful results, it is essential that all solute be dissolved completely prior to the addition of ice. Continuous swirling or stirring is sufficient, but it may take a few minutes for some of the salts. Waste Handling: Ferric nitrate solutions are collected as hazardous waste - a 4L jug is provided in Waste Hood All other solutions may be flushed down the drain with plenty of water Procedure 1. Fill a 250 ml beaker with about 50 mL of water and crushed ice to make a slurry. Place the beaker with the ice slurry on the stir plate. Select "Collect" on the laptop screen, and immediately lower the temperature probe into the mixture. The program will record a temperature reading every 0.5 seconds for 4 minutes. Use the lowest temperature recorded. a. If the lowest temperature is not 0.0C, a correction will be needed in the calculations to correct for errors in subsequent measurements. 2. Empty the beaker and rinse & dry it thoroughly for future use. There are (3) 250 ml beakers in the lab drawer. Clean the used ones as you go during the 4 minutes of waiting for the temperature recordings. 3. Tare a clean, empty 250 ml beaker. Add and Record the mass of solute required. The known solute masses are specified below. Each unknown is labelled with the mass required as well as the van't Hoff factor. a. NaCl (sodium chloride) - 2.91g b. C12H2O11 (sucrose) - 17.118 c. CaCl 2 H2O (calcium chloride dihydrate) - 7.358 d. Fe(NO))*9 H20 (ferric nitrate nonahydrate) 20.198 4. Tare the beaker + solute. Remove the balance from the beaker and add 50 mL of Ro water to the solute in the beaker Modified Freezing Point Depression Experiment 5. On the tared balance, weigh the beakersolute. water. Record the mass of the 50 ml water. Add the clean stir bar to the solution 5. Stir or swirl the solution until the solute is completely dissolved. You may use the stirplate for this step. All solid must be dissolved before moving on 7. Tare a 100 ml beaker. Add 50 g of ice to the beaker recording the exact mass. 8. Pour the ice into the 250 ml beaker with the solution and immediately lower the temperature probe into the mixture. Press Collect on the laptop screen. Make sure all the ice goes into the beaker and that the temperature probe is not touching the beaker. 9. Swirl ice/solution mixture for 5 seconds to accelerate mixing, then allow to stir undisturbed at 300 rpm for the remainder of the program run time. 10. One the four-minute program ends, verify there is still ice in the mixture. If there is, record the lowest temperature obtained for the mixture. (As long as there is ice remaining the solution is still at its freezing point.) Consult your GTA if there is no ice present. 11. Dispose of the solution as specified in "Waste Handling : Rinse the probe thoroughly with RO water and dry. Dry all glassware thoroughly before using again. 12. Repeat Steps 3 through 11 with each remaining salt and the assigned unknown sample. You will perform this procedure 6 times in total, Calculations Note: Use the carbon copy pages in the back of your manual if you need more room to show your calculations. These will be turned in with your report. Calculate the molality of each of known solution Calculate the theoretical freezing point of each known solution Calculate the error of the observed FP from the theoretical FP calculated Calculate the experimentally determined AT, for each unknown analysis. Use the experimentally determined AT in calculated the molecular weight of the unknown sample Report the average molecular weight determined. Remember: moles = a/MW thnak u 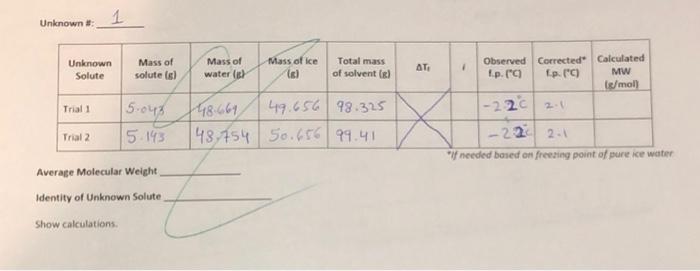








Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


