Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help with the calculations D. Hont of solution Now, imagine that, rather than the mass M being dropped into the calorimeter cold water, NH.Cs)
need help with the calculations 


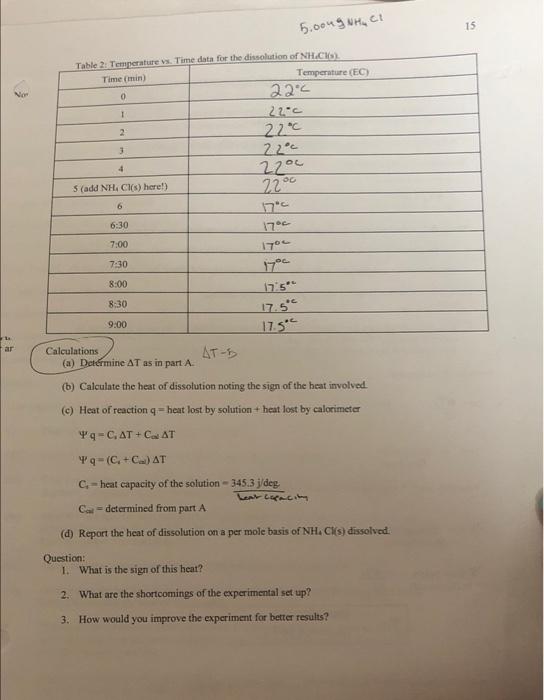
D. Hont of solution Now, imagine that, rather than the mass M being dropped into the calorimeter cold water, NH.Cs) is dropped. One can determine the heat accompanying the ensuing dissolution since the heat capacity of the calorimeter is now known (see Cabove). The heat involved in dissolution, q - heat gained by solution + heat gained by calorimeter "If the dissolution is endothermic, then appropriate signs must be employed to account for the Aendothermicity The heat of the solution - CAT The heat of the calorimeter Cp, AT Therefore, the heat of dissolution, q will be 4-C.(TBT) Ops (TBT) Where Tiand Tiare the initial and final temperatures of the calorimeter and water, respectively. C - heat capacity of the solution 4.3 The calorimeter In this experiment your calorimeter will be a coffee cup placed in a beaker and covered with styrofoam or any other insulating material containing a hole at the top for the insertion of a thermometer. This thermometer will serve simultaneously as a temperature measuring device and a stirrer. 4.4 Procedure A. Heat capacity of the calorimeter 0 Pour 50.0 ml. of cold water into the calorimeter (ii) Insert the thermometer in the cover and place on top of the calorimeter. Wait five minutes with periodic stirring. During this wait period, measure 50.0 ml of 70 degrees C water in a 100 ml graduated cylinder After the five minutes wait period begin taking temperature measurements at one minute intervals for another five minutes. Be sure to record the temperatures and times during this time period (five minutes). (v) Again, take the temperature of the hot water and record it in a safe place. (vi) Quickly pour the hot water into the calorimeter and continue to read the temperature every thirty (30) seconds for another five minutes (iv) This should be continuous from the previous five minutes in (iv); i.e, there should be no break in timing from the initial temperature measurements involving cold water as described in (iv) (see table) Keep your calorimeter and accessories in a safe place for the next laboratory period when you will determine the enthalpy of the dissolution of NH. C (s). (vii) 13 Table 1. Temperature vs Time Time (min) 1 2 3 4 Hor word 13 S (Add hot water here!) 6 7 7:30 Temperature (EC) 22 C 22 C 22 C 22 *C 22 C 47.5C 466 46C 45ic 45 44c 44c 43.5c 43c 430 8:00 8.30 9:00 9:30 10:00 10:30 11:00 Calculations: (a) Make a plot of temperature vs. time in minutes. You should obtain a sigmoid or reverse sigmoid curve depending upon whether the reaction is exothermic or endothermic. AT 21 (b) Your instructor will help you to determine AT. This will be the change in temperature for the cold wafer and the calorimeter (C) Heat lost by hot water is 4 - Blu (TBT Where To maximum temperature (reached after mixing) and Ti-initial temperature of the hot water just before it is poured into the calorimeter (see 4.4 V above) $ From the volume of the hot water, calculate the mass assurning the density of water to be 1.00 g/ml $ Calculate the number of moles of hot water lu using this mass calculated and the molecular wt of water $ Can of water is obtained from your text book. 14 heat caracity (d) heat gained by cold water is Solgt 0.- (AT) Where is a discussed with your instructor and Co. is molar beat capacity of water (e) heat gained by calorimeter and accessory, the thermometer, is -CAT ( 14.9 Pan (TBT) nCAT Coal AT Yen (TYBT) in C+C) AT YB (TBT) B n.CC AT "You cannot have a negative heat capacity. If you do, check and recheck your calculation or ask for your instructors help! "Your C value should be 5.997 5 deg for the calorimeter provided. hear lose by Solution - heat gained by Calbromence? O exo hemie beltet. Jan 1:)) (g) Calculate the error B. Hent of dissolution of N.C.15 enemem 0 Measure and pour 50.0mL of distilled water in the dry calorimeter. (m) Wait five minutes. During this wait period weigh 5.000 g of NH.Ch, and keep in a safe place. (in) After the five minutes wait.start temperature time measurements at one minute interval for five minutes (iv) Quickly open the calorimeter at the fifth minute and pour all the 5,000g of NHCl(s) into the wate (v) Close and stir vigorously and continue temperature time measurements every 30 seconds for another three minutes. (vi) Make a temperature vs. time plot as in part of this procedure. -(olto-T11 + ((14-T.) 5.00ug NhuCU 15 Table 2. Temperature vs. Time data for the dissolution of NH.CH Time min) Temperature (EC) 0 22C 1 22.c 2 2200 3 22C 4 22ou (add NH, Cl(s) here!) 22 6 17 6:30 1700 7:00 17. 7:30 8:00 17 17.5 17.5C 17.5" 8:30 9:00 Calculations (a) Determine AT as in part A. ATE (b) Calculate the heat of dissolution noting the sign of the heat involved (c) Heat of reaction - heat lost by solution - heat lost by calorimeter q-C, AT+CHAT 49-(C, +C) AT Cheat capacity of the solution - 345.3 jdeg heat capacity Ca determined from part A (d) Report the heat of dissolution on a per mole basis of NH. Cs) dissolved. Question: 1. What is the sign of this heat? 2. What are the shortcomings of the experimental set up? 3. How would you improve the experiment for better results? D. Hont of solution Now, imagine that, rather than the mass M being dropped into the calorimeter cold water, NH.Cs) is dropped. One can determine the heat accompanying the ensuing dissolution since the heat capacity of the calorimeter is now known (see Cabove). The heat involved in dissolution, q - heat gained by solution + heat gained by calorimeter "If the dissolution is endothermic, then appropriate signs must be employed to account for the Aendothermicity The heat of the solution - CAT The heat of the calorimeter Cp, AT Therefore, the heat of dissolution, q will be 4-C.(TBT) Ops (TBT) Where Tiand Tiare the initial and final temperatures of the calorimeter and water, respectively. C - heat capacity of the solution 4.3 The calorimeter In this experiment your calorimeter will be a coffee cup placed in a beaker and covered with styrofoam or any other insulating material containing a hole at the top for the insertion of a thermometer. This thermometer will serve simultaneously as a temperature measuring device and a stirrer. 4.4 Procedure A. Heat capacity of the calorimeter 0 Pour 50.0 ml. of cold water into the calorimeter (ii) Insert the thermometer in the cover and place on top of the calorimeter. Wait five minutes with periodic stirring. During this wait period, measure 50.0 ml of 70 degrees C water in a 100 ml graduated cylinder After the five minutes wait period begin taking temperature measurements at one minute intervals for another five minutes. Be sure to record the temperatures and times during this time period (five minutes). (v) Again, take the temperature of the hot water and record it in a safe place. (vi) Quickly pour the hot water into the calorimeter and continue to read the temperature every thirty (30) seconds for another five minutes (iv) This should be continuous from the previous five minutes in (iv); i.e, there should be no break in timing from the initial temperature measurements involving cold water as described in (iv) (see table) Keep your calorimeter and accessories in a safe place for the next laboratory period when you will determine the enthalpy of the dissolution of NH. C (s). (vii) 13 Table 1. Temperature vs Time Time (min) 1 2 3 4 Hor word 13 S (Add hot water here!) 6 7 7:30 Temperature (EC) 22 C 22 C 22 C 22 *C 22 C 47.5C 466 46C 45ic 45 44c 44c 43.5c 43c 430 8:00 8.30 9:00 9:30 10:00 10:30 11:00 Calculations: (a) Make a plot of temperature vs. time in minutes. You should obtain a sigmoid or reverse sigmoid curve depending upon whether the reaction is exothermic or endothermic. AT 21 (b) Your instructor will help you to determine AT. This will be the change in temperature for the cold wafer and the calorimeter (C) Heat lost by hot water is 4 - Blu (TBT Where To maximum temperature (reached after mixing) and Ti-initial temperature of the hot water just before it is poured into the calorimeter (see 4.4 V above) $ From the volume of the hot water, calculate the mass assurning the density of water to be 1.00 g/ml $ Calculate the number of moles of hot water lu using this mass calculated and the molecular wt of water $ Can of water is obtained from your text book. 14 heat caracity (d) heat gained by cold water is Solgt 0.- (AT) Where is a discussed with your instructor and Co. is molar beat capacity of water (e) heat gained by calorimeter and accessory, the thermometer, is -CAT ( 14.9 Pan (TBT) nCAT Coal AT Yen (TYBT) in C+C) AT YB (TBT) B n.CC AT "You cannot have a negative heat capacity. If you do, check and recheck your calculation or ask for your instructors help! "Your C value should be 5.997 5 deg for the calorimeter provided. hear lose by Solution - heat gained by Calbromence? O exo hemie beltet. Jan 1:)) (g) Calculate the error B. Hent of dissolution of N.C.15 enemem 0 Measure and pour 50.0mL of distilled water in the dry calorimeter. (m) Wait five minutes. During this wait period weigh 5.000 g of NH.Ch, and keep in a safe place. (in) After the five minutes wait.start temperature time measurements at one minute interval for five minutes (iv) Quickly open the calorimeter at the fifth minute and pour all the 5,000g of NHCl(s) into the wate (v) Close and stir vigorously and continue temperature time measurements every 30 seconds for another three minutes. (vi) Make a temperature vs. time plot as in part of this procedure. -(olto-T11 + ((14-T.) 5.00ug NhuCU 15 Table 2. Temperature vs. Time data for the dissolution of NH.CH Time min) Temperature (EC) 0 22C 1 22.c 2 2200 3 22C 4 22ou (add NH, Cl(s) here!) 22 6 17 6:30 1700 7:00 17. 7:30 8:00 17 17.5 17.5C 17.5" 8:30 9:00 Calculations (a) Determine AT as in part A. ATE (b) Calculate the heat of dissolution noting the sign of the heat involved (c) Heat of reaction - heat lost by solution - heat lost by calorimeter q-C, AT+CHAT 49-(C, +C) AT Cheat capacity of the solution - 345.3 jdeg heat capacity Ca determined from part A (d) Report the heat of dissolution on a per mole basis of NH. Cs) dissolved. Question: 1. What is the sign of this heat? 2. What are the shortcomings of the experimental set up? 3. How would you improve the experiment for better results 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


