Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need help woth limiting agent and Therptical yield havent don this in years struggling to solve this with limited info 4. Determine the limiting reactant,
need help woth limiting agent and Therptical yield havent don this in years struggling to solve this with limited info 
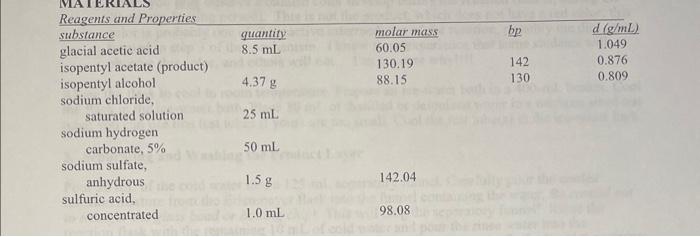
4. Determine the limiting reactant, and the calculate the theoretical yield of isopentyl acetate, in grams, for the esterification reaction described in the Procedure. Note that the density of glacial acetic acid is provided in the Reagents table as 1.049g/mL. The overall balanced reaction is shown in Equation 7 at the bottom of page 3. You must show your work to receive credit on this question. (Note: you may wish to make a copy of this calculation, because you will also need it for your post-lab questions). Reagents and Properties substanceglacialaceticacidisopentylacetate(product)isopentylalcoholquantity8.5mL4.37gmolarmass60.05130.1988.15bp1421301.049d(g/mL)0.8760.809 sodium chloride, saturated solution 25mL sodium hydrogen carbonate, 5%50mL sodium sulfate, anhydrous 1.5g 142.04 sulfuric acid, concentrated 1.0mL 98.08 

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


