Answered step by step
Verified Expert Solution
Question
1 Approved Answer
need hep with post lab esterfication problems. the mass recorded of my isopentyl acetate was 29.76 MATERIALS Reagents and Properties substanceglacialaceticacidisopentylacetate(product)isopentylalcoholquantity8.5mL4.37gmolarmass60.05130.1988.15bp1421301.049d(g/mL)0.8760.809 sodium chloride, saturated solution
need hep with post lab esterfication problems. the mass recorded of my isopentyl acetate was 29.76 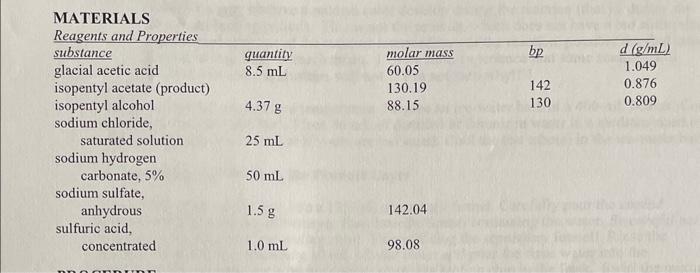

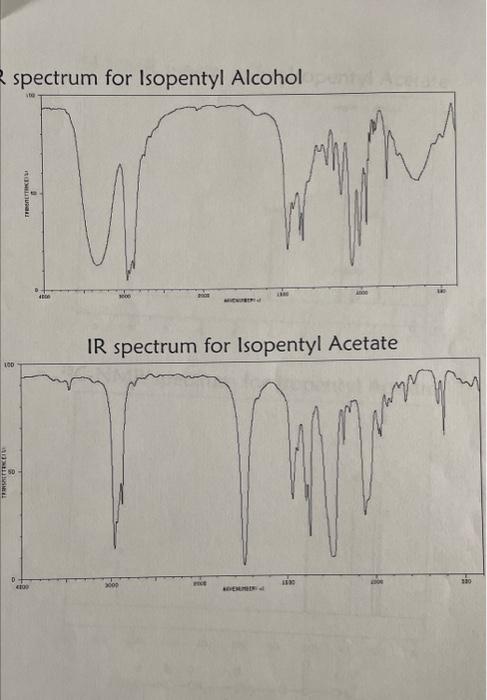
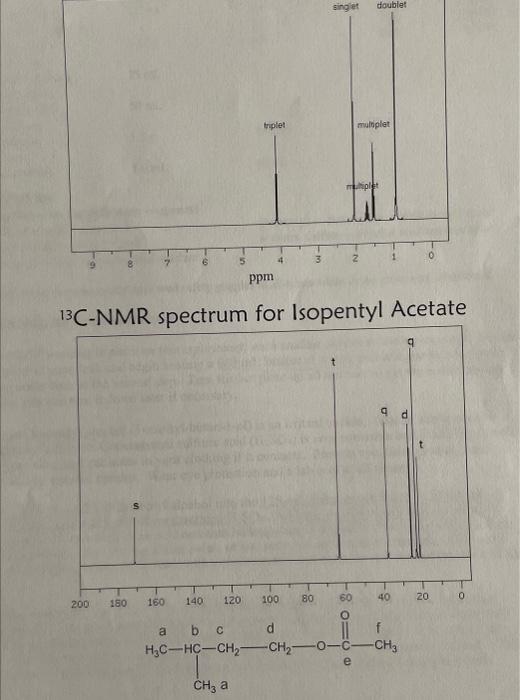
MATERIALS Reagents and Properties substanceglacialaceticacidisopentylacetate(product)isopentylalcoholquantity8.5mL4.37gmolarmass60.05130.1988.15bp1421301.049d(g/mL)0.8760.809 sodium chloride, saturated solution 25mL sodium hydrogen carbonate, 5%50mL sodium sulfate, anhydrous1.5g142.04 sulfuric acid, concentrated1.0mL98.08 1. Based on the quantities of chemicals you used, determine the theoretical yield of isopentyl acetate in grams, and the limiting reactant in this experiment. Calculate your percent yield of isopentyl acetate. You MUST show your work, including calculation of theoretical yield, to receive credit on this question. 2. The IR spectra for isopentyl alcohol and isopentyl acetate are given on the next page. List the distinctive features of the IR spectra of isopentyl acetate, as well as isopentyl alcohol and acetic acid if provided. Assign the absorption bands to the appropriate functional groups. What are the principal differences between the IR spectra for the reactant and product? 3. Fully analyze the 1HNMR and 13CNMR spectra for your product, isopentyl acetate, which are provided on the following pages. I have provided the structure of the product at the bottom of the page, with the carbons (and their corresponding hydrogens) pre-labeled as " a ", " b ", " c ", etc. Label the corresponding peaks in the two spectra with the same letter, as with many examples in class and in the book. (Note there will not be a hydrogen group " e " as that carbon doesn't have any hydrogens on it). 4. a. Write the equation for the reaction of 5%NaHCO3 with acetic acid. b. Why does the reaction mixture release CO2 when it is neutralized with NaHCO3 ? Where does the CO2 come from? 5. What is the IUPAC name for isopentyl acetate? spectrum for Isopentvl Alcohol ID cnortrium far Ichnontul Acetate 13r NIMA cnertrum for Isopentyl Acetate 



Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


