Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Need only 2 and 3, time is t=28.125 hrs for 1.! Problem 4-11 (Level 2) The organic acid, ACOOH, reacts reversibly with the alcohol, BOH,
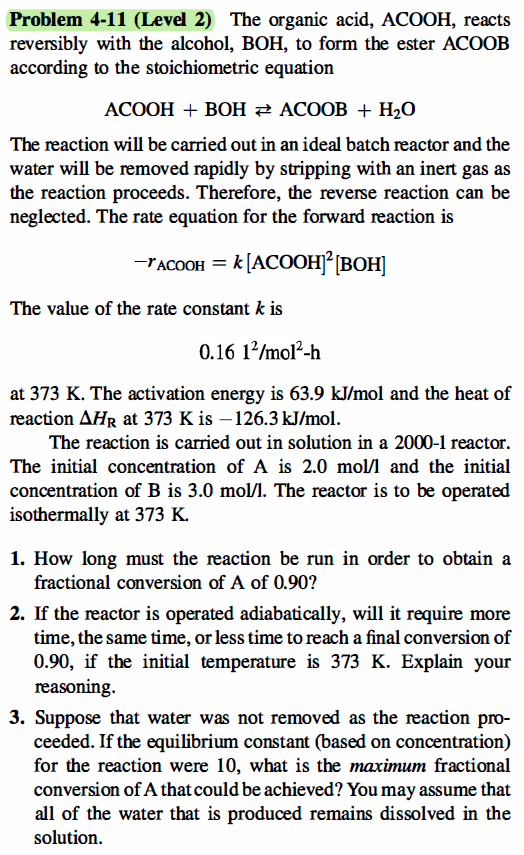
Need only 2 and 3, time is t=28.125 hrs for 1.!
Problem 4-11 (Level 2) The organic acid, ACOOH, reacts reversibly with the alcohol, BOH, to form the ester ACOOB according to the stoichiometric equation ACOOH + BOH E ACOOB + H20 The reaction will be carried out in an ideal batch reactor and the water will be removed rapidly by stripping with an inert gas as the reaction proceeds. Therefore, the reverse reaction can be neglected. The rate equation for the forward reaction is -racooh = k [ACOOH][BOH] The value of the rate constant k is 0.16 12/mol-1 at 373 K. The activation energy is 63.9 kJ/mol and the heat of reaction AHR at 373 K is 126.3 kJ/mol. The reaction is carried out in solution in a 2000-1 reactor. The initial concentration of A is 2.0 mol/l and the initial concentration of B is 3.0 mol/l. The reactor is to be operated isothermally at 373 K. 1. How long must the reaction be run in order to obtain a fractional conversion of A of 0.90? 2. If the reactor is operated adiabatically, will it require more time, the same time, or less time to reach a final conversion of 0.90, if the initial temperature is 373 K. Explain your reasoning 3. Suppose that water was not removed as the reaction pro- ceeded. If the equilibrium constant (based on concentration) for the reaction were 10, what is the maximum fractional conversion of A that could be achieved? You may assume that all of the water that is produced remains dissolved in the solutionStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


