Question
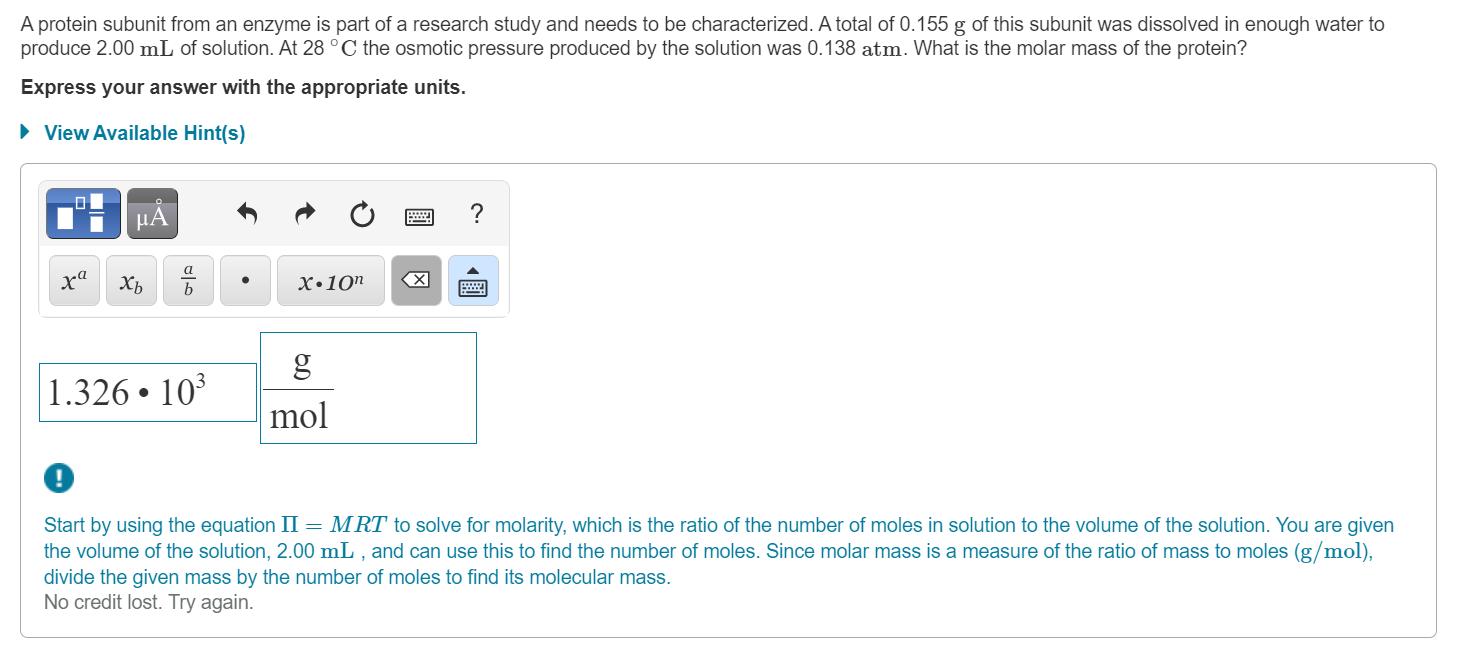
A protein subunit from an enzyme is part of a research study and needs to be characterized. A total of 0.155 g of this
A protein subunit from an enzyme is part of a research study and needs to be characterized. A total of 0.155 g of this subunit was dissolved in enough water to produce 2.00 mL of solution. At 28 C the osmotic pressure produced by the solution was 0.138 atm. What is the molar mass of the protein? Express your answer with the appropriate units. View Available Hint(s) A xa X10" X 1.326 10 mol Start by using the equation II = MRT to solve for molarity, which is the ratio of the number of moles in solution to the volume of the solution. You are given the volume of the solution, 2.00 mL , and can use this to find the number of moles. Since molar mass is a measure of the ratio of mass to moles (g/mol), divide the given mass by the number of moles to find its molecular mass. No credit lost. Try again.
Step by Step Solution
3.38 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
General Chemistry
Authors: Darrell Ebbing, Steven D. Gammon
9th edition
978-0618857487, 618857486, 143904399X , 978-1439043998
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App