
NEEDED INFORMATION:
Kinetic Run 1
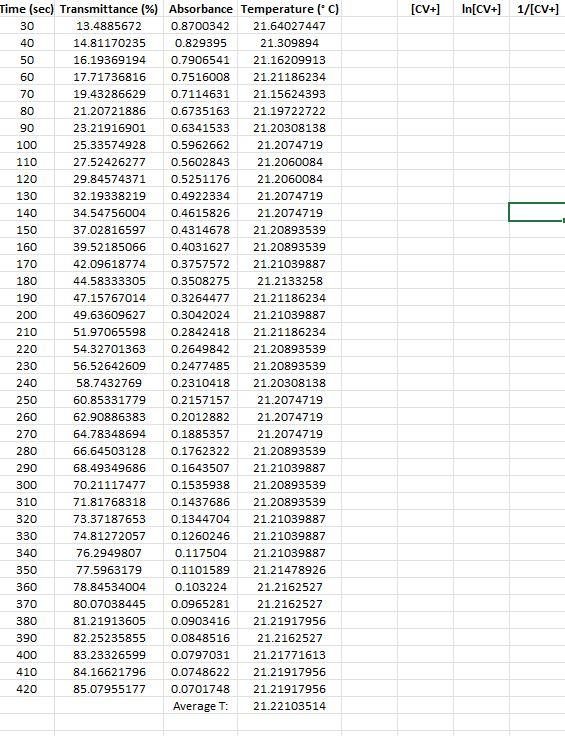 Kinetic Run 2
Kinetic Run 2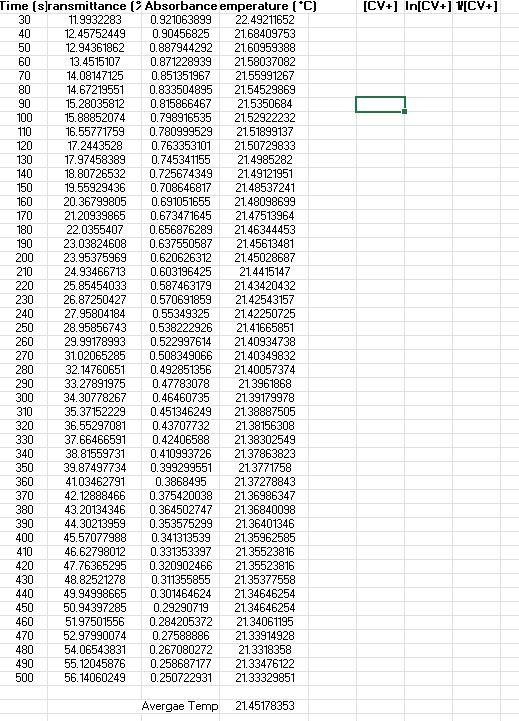
Kinetic Run 3:
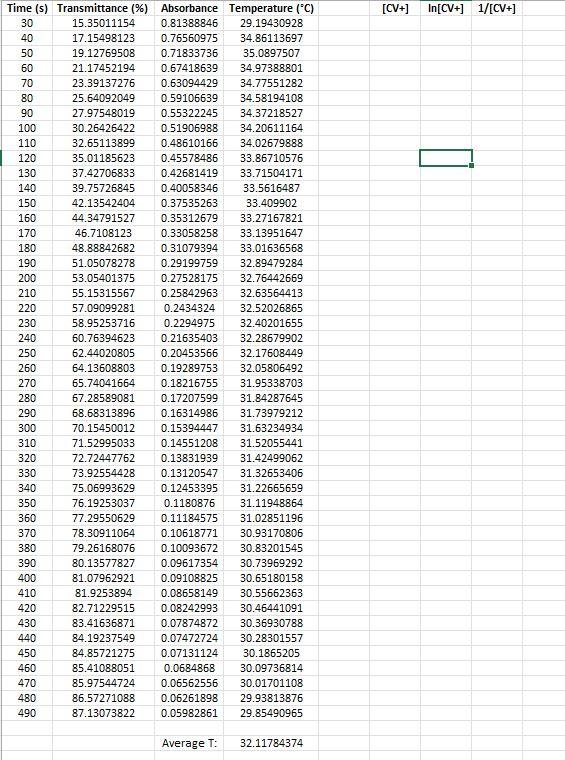
88 2. Now we must make a series of dilutions of a known concentration of CV+ solution. Using the proper glassware is essential because we must know the exact concentration of each dilution. On your Response Sheet, describe how you would make dilutions of CV+. Specifically, describe how you would use a stock solution of CV+ and specialized glassware to make 4 test tubes containing 10.00mL each (as 1:10, 2:10, 4:10, and 8:10 dilutions). The more descriptive you are, the better. 3. Now we can obtain the data necessary to make a calibration curve. We can take absorbance measurements by aliquoting a small volume of each diluted solution of CV+ of known concentration into 4 cuvettes and place them (one at a time) in our calibrated colorimeter. We obtain the following data: Concentration Absorbance 2.00 x 10M 0.125 5.00 x 10M 0.313 1.00 x 105M 0.612 2.00 x 10M 0.961 No chemical reaction is occurring, so this part of the experiment is not time sensitive. The CV+ has not been mixed with hydroxide ions yet. Before we run our first kinetic experiment, it is important to make sure our measurements are reasonable. Use the data provided above and generate am XY-scatter plot in Microsoft Excel or a similar program. Make sure your graph is consistent with the Beer-Lambert Law discussed in the introduction. Your graph should include a best-fit trendline, equation for that line, and correlation coefficient (R). It should also be neat, have a proper title including your name, and the axes should be labeled and contain any applicable units. This graph is your calibration curve. Attach it to your Response Sheet when you submit your work. 4. The next portion of the experiment is the point at which we combine different concentrations of CV+ with an excess of OH- in solution. Varying the initial concentration of CV+ (or (CV+).) will allow us to see how (CV+] affects the rate of the reaction. Running one of these experiments at a higher temperature will provide us with the data necessary to also determine the activation energy of the reaction. Each one of these kinetic experiments are performed by combining a known volume of known concentration of CV+ with a known volume of known concentration of NaOH solution in a carefully calculated way. The (CV+), must be precisely calculated by determining the moles of CV+added, and then dividing those moles by the new total volume of the reaction mixture. This part of the experiment is time sensitive! As soon as the reactants are mixed, one must aliquot a sample into a cuvette and place it in the colorimeter. Measurement by the colorimeter must be initiated at the same time as mixing reactants because this is the time at which the reaction begins. Once the cuvette is placed in the colorimeter, absorbance data is collected in 10s intervals until the reaction is complete. The first few data points are not recorded because the sample in the cuvette has not yet been inserted into the colorimeter. Once the sample in the cuvette has been inserted into the colorimeter, we can monitor the reaction and watch the data points accumulate as plotted on a graph. The reaction is Pre-Lab Questions 1. Examine the images of test tubes in the Pre-Lab Concept Check. Which tube contains a more concentrated sample of crystal violet? Choose test tube A or B and explain your choice using complete sentences. Assume all conditions other than concentration are equal between the two test tube images (i.e., lighting, test tube size, etc.). 2. Which tube would give a lower percent transmittance %T) measurement and why? 3. Which tube would give a lower absorbance (A) measurement and why? 4. Discard the assumptions made in the previous questions. If the images of the two test tubes containing CV+ had the same concentration (photographed under identical ambient lighting conditions), provide an explanation for the difference between the two images? Hint: Path length (b) is the distance light must travel to get through a sample. Experimental Summary Questions Step 2. Describe how you would make dilutions of CV+. Specifically, describe how you would use a stock solution of CV+ and specialized glassware to make 4 test tubes containing 10.00mL each (as 1:10, 2:10,4:10, and 8:10 dilutions). The more descriptive you are, the better. Step 3. Generate a calibration curve as outlined in the Experimental Summary. Post-Lab Questions 1. Generate the nine plots as outlined in the Data Analysis section above. 2. Using your graphs, determine if the reaction is zero, first, or second order with respect to the concentration of crystal violet? Keep in mind you are using experimental data so some variation will exist, but you should still be able to determine reaction order for (CV+). Explain your choice citing your data/graphs. 3. Calculate the rate constant (k) using the slope of the most linear curve for the 21C kinetic runs and the ~32C kinetic run. Keep in mind that rate constant k = (-1)slope for 0 and 1 reactions and k = slope for 2 reactions. 4. Write the correct rate law equation for the reaction with respect to crystal violet. You may omit (OH) from the rate law. 5. Calculate the activation energy for the reaction performed in this exercise. [CV+) In[CV+) 1/[CV+] 90 Time (sec) Transmittance %) Absorbance Temperature (C) 30 13.4885672 0.8700342 21.64027447 40 14.81170235 0.829395 21.309894 50 16.19369194 0.7906541 21.16209913 60 17.71736816 0.7516008 21.21186234 70 19.43286629 0.7114631 21.15624393 80 21.20721886 0.6735163 21.19722722 23.21916901 0.6341533 21.20308138 100 25.33574928 0.5962662 21.2074719 110 27.52426277 0.5602843 21.2060084 120 29.84574371 0.5251176 21.2060084 130 32.19338219 0.4922334 21.2074719 140 34.54756004 0.4615826 21.2074719 150 37.02816597 0.4314678 21.20893539 160 39.52185066 0.4031627 21.20893539 170 42.09618774 0.3757572 21.21039887 180 44.58333305 0.3508275 21.2133258 190 47.15767014 0.3264477 21.21186234 200 49.63609627 0.3042024 21.21039887 210 51.97065598 0.2842418 21.21186234 220 54.32701363 0.2649842 21.20893539 230 56.52642609 0.2477485 21.20893539 240 58.7432769 0.2310418 21.20308138 250 60.85331779 0.2157157 21.2074719 260 62.90886383 0.2012882 21.2074719 270 64.78348694 0.1885357 21.2074719 280 66.64503128 0.1762322 21.20893539 290 68.49349686 0.1643507 21.21039887 300 70.21117477 0.1535938 21.20893539 310 71.81768318 0.1437686 21.20893539 320 73.37187653 0.1344704 21.21039887 330 74.81272057 0.1260246 21.21039887 340 76.2949807 0.117504 21.21039887 350 77.5963179 0.1101589 21.21478926 360 78.84534004 0.103224 21.2162527 370 80.07038445 0.0965281 21.2162527 380 81.21913605 0.0903416 21.21917956 390 82.25235855 0.0848516 21.2162527 400 83.23326599 0.0797031 21.21771613 410 84.16621796 0.0748622 21.21917956 420 85.07955177 0.0701748 21.21917956 Average : 21.22103514 [CY+] In[CV+] W[CY+] Time (s]ransmittance (? Absorbance emperature (C) 30 11.9932283 0.921063899 22.49211652 40 12.45752449 0.90456825 21.68409753 50 12.94361862 0.887944292 21.60959388 60 13.4515107 0.871228939 21.58037082 70 14.08147125 0.851351967 21.55991267 80 14.67219551 0.833504895 21.54529869 90 15.28035812 0.815866467 21.5350684 100 15.88852074 0.798916535 21.52922232 110 16.55771759 0.780999529 21.51899137 120 17.2443528 0.763353101 21.50729833 130 17.97458389 0.745341155 21.4985282 140 18.80726532 0.725674349 21.49121951 150 19.55929436 0.708646817 21.48537241 160 20.36799805 0.691051655 21.48098699 170 21.20939865 0.673471645 21.47513964 180 22.0355407 0.656876289 21.46344453 190 23.03824608 0.637550587 21.45613481 200 23.95375969 0.620626312 21.45028687 210 24.93466713 0.603196425 21.4415147 220 25.85454033 0.587463179 21.43420432 230 26.87250427 0.570691859 21.42543157 240 27.95804184 0.55349325 21.42250725 250 28.95856743 0.538222926 21.41665851 260 29.99178993 0.522997614 21.40934738 270 31.02065285 0.508349066 21.40349832 280 32.14760651 0.492851356 21.40057374 290 33.27891975 0.47783078 21.3961868 300 34.30778267 0.46460735 21.39179978 310 35.37152229 0.451346249 21.38887505 320 36.55297081 0.43707732 21.38156308 330 37.66466591 0.42406588 21.38302549 340 38.81559731 0.410993726 21.37863823 350 39.87497734 0.399299551 21.3771758 360 41.03462791 0.3868495 21.37278843 370 42.12888466 0.375420038 21.36986347 380 43.20134346 0.364502747 21.36840098 390 44.30213959 0.353575299 21.36401346 400 45.57077988 0.341313539 21.35962585 410 46.62798012 0.331353397 21.35523816 420 47.76365295 0.320902466 21.35523816 430 48.82521278 0.311355855 21.35377558 440 49.94998665 0.301464624 21.34646254 450 50.94397285 0.29290719 21.34646254 460 51.97501556 0.284205372 21.34061195 470 52.97990074 0.27588886 21.33914928 480 54.06543831 0.267080272 21.3318358 490 55. 12045876 0.258687177 21.33476122 500 56.14060249 0.250722931 21.33329851 Avergae Temp 21.45178353 [CV+] In[CV+ 1/[CV+) Time (s) Transmittance (%) Absorbance Temperature (C) 30 15.35011154 0.81388846 29.19430928 40 17.15498123 0.76560975 34.86113697 50 19.12769508 0.71833736 35.0897507 60 21.17452194 0.67418639 34.97388801 70 23.39137276 0.63094429 34.77551282 80 25.64092049 0.59106639 34.58194108 90 27.97548019 0.55322245 34.37218527 100 30.26426422 0.51906988 34.20611164 110 32.65113899 0.48610166 34.02679888 120 35.01185623 0.45578486 33.86710576 130 37.42706833 0.42681419 33.71504171 140 39.75726845 0.40058346 33.5616487 150 42.13542404 0.37535263 33.409902 160 44.34791527 0.35312679 33.27167821 170 46.7108123 0.33058258 33.13951647 180 48.88842682 0.31079394 33.01636568 190 51.05078278 0.29199759 32.89479284 200 53.05401375 0.27528175 32.76442669 210 55.15315567 0.25842963 32.63564413 220 57.09099281 0.2434324 32.52026865 230 58.95253716 0.2294975 32.40201655 240 60.76394623 0.21635403 32.28679902 250 62.44020805 0.20453566 32.17608449 260 64.13608803 0.19289753 32.05806492 270 65.74041664 0.18216755 31.95338703 280 67.28589081 0.17207599 31.84287645 290 68.68313896 0.16314986 31.73979212 300 70.15450012 0.15394447 31.63234934 310 71.52995033 0.14551208 31.52055441 320 72.72447762 0.13831939 31.42499062 330 73.92554428 0.13120547 31.32653406 340 75.06993629 0.12453395 31.22665659 350 76.19253037 0.1180876 31.11948864 360 77.29550629 0.11184575 31.02851196 370 78.30911064 0.10618771 30.93170806 380 79.26168076 0.10093672 30.83201545 390 80.13577827 0.09617354 30.73969292 400 81.07962921 0.09108825 30.65180158 410 81.9253894 0.08658149 30.55662363 420 82.71229515 0.08242993 30.46441091 430 83.41636871 0.07874872 30.36930788 440 84.19237549 0.07472724 30.28301557 450 84.85721275 0.07131124 30.1865205 460 85.41088051 0.0684868 30.09736814 470 85.97544724 0.06562556 30.01701108 480 86.57271088 0.06261898 29.93813876 490 87.13073822 0.05982861 29.85490965 Average T: 32.11784374 88 2. Now we must make a series of dilutions of a known concentration of CV+ solution. Using the proper glassware is essential because we must know the exact concentration of each dilution. On your Response Sheet, describe how you would make dilutions of CV+. Specifically, describe how you would use a stock solution of CV+ and specialized glassware to make 4 test tubes containing 10.00mL each (as 1:10, 2:10, 4:10, and 8:10 dilutions). The more descriptive you are, the better. 3. Now we can obtain the data necessary to make a calibration curve. We can take absorbance measurements by aliquoting a small volume of each diluted solution of CV+ of known concentration into 4 cuvettes and place them (one at a time) in our calibrated colorimeter. We obtain the following data: Concentration Absorbance 2.00 x 10M 0.125 5.00 x 10M 0.313 1.00 x 105M 0.612 2.00 x 10M 0.961 No chemical reaction is occurring, so this part of the experiment is not time sensitive. The CV+ has not been mixed with hydroxide ions yet. Before we run our first kinetic experiment, it is important to make sure our measurements are reasonable. Use the data provided above and generate am XY-scatter plot in Microsoft Excel or a similar program. Make sure your graph is consistent with the Beer-Lambert Law discussed in the introduction. Your graph should include a best-fit trendline, equation for that line, and correlation coefficient (R). It should also be neat, have a proper title including your name, and the axes should be labeled and contain any applicable units. This graph is your calibration curve. Attach it to your Response Sheet when you submit your work. 4. The next portion of the experiment is the point at which we combine different concentrations of CV+ with an excess of OH- in solution. Varying the initial concentration of CV+ (or (CV+).) will allow us to see how (CV+] affects the rate of the reaction. Running one of these experiments at a higher temperature will provide us with the data necessary to also determine the activation energy of the reaction. Each one of these kinetic experiments are performed by combining a known volume of known concentration of CV+ with a known volume of known concentration of NaOH solution in a carefully calculated way. The (CV+), must be precisely calculated by determining the moles of CV+added, and then dividing those moles by the new total volume of the reaction mixture. This part of the experiment is time sensitive! As soon as the reactants are mixed, one must aliquot a sample into a cuvette and place it in the colorimeter. Measurement by the colorimeter must be initiated at the same time as mixing reactants because this is the time at which the reaction begins. Once the cuvette is placed in the colorimeter, absorbance data is collected in 10s intervals until the reaction is complete. The first few data points are not recorded because the sample in the cuvette has not yet been inserted into the colorimeter. Once the sample in the cuvette has been inserted into the colorimeter, we can monitor the reaction and watch the data points accumulate as plotted on a graph. The reaction is Pre-Lab Questions 1. Examine the images of test tubes in the Pre-Lab Concept Check. Which tube contains a more concentrated sample of crystal violet? Choose test tube A or B and explain your choice using complete sentences. Assume all conditions other than concentration are equal between the two test tube images (i.e., lighting, test tube size, etc.). 2. Which tube would give a lower percent transmittance %T) measurement and why? 3. Which tube would give a lower absorbance (A) measurement and why? 4. Discard the assumptions made in the previous questions. If the images of the two test tubes containing CV+ had the same concentration (photographed under identical ambient lighting conditions), provide an explanation for the difference between the two images? Hint: Path length (b) is the distance light must travel to get through a sample. Experimental Summary Questions Step 2. Describe how you would make dilutions of CV+. Specifically, describe how you would use a stock solution of CV+ and specialized glassware to make 4 test tubes containing 10.00mL each (as 1:10, 2:10,4:10, and 8:10 dilutions). The more descriptive you are, the better. Step 3. Generate a calibration curve as outlined in the Experimental Summary. Post-Lab Questions 1. Generate the nine plots as outlined in the Data Analysis section above. 2. Using your graphs, determine if the reaction is zero, first, or second order with respect to the concentration of crystal violet? Keep in mind you are using experimental data so some variation will exist, but you should still be able to determine reaction order for (CV+). Explain your choice citing your data/graphs. 3. Calculate the rate constant (k) using the slope of the most linear curve for the 21C kinetic runs and the ~32C kinetic run. Keep in mind that rate constant k = (-1)slope for 0 and 1 reactions and k = slope for 2 reactions. 4. Write the correct rate law equation for the reaction with respect to crystal violet. You may omit (OH) from the rate law. 5. Calculate the activation energy for the reaction performed in this exercise. [CV+) In[CV+) 1/[CV+] 90 Time (sec) Transmittance %) Absorbance Temperature (C) 30 13.4885672 0.8700342 21.64027447 40 14.81170235 0.829395 21.309894 50 16.19369194 0.7906541 21.16209913 60 17.71736816 0.7516008 21.21186234 70 19.43286629 0.7114631 21.15624393 80 21.20721886 0.6735163 21.19722722 23.21916901 0.6341533 21.20308138 100 25.33574928 0.5962662 21.2074719 110 27.52426277 0.5602843 21.2060084 120 29.84574371 0.5251176 21.2060084 130 32.19338219 0.4922334 21.2074719 140 34.54756004 0.4615826 21.2074719 150 37.02816597 0.4314678 21.20893539 160 39.52185066 0.4031627 21.20893539 170 42.09618774 0.3757572 21.21039887 180 44.58333305 0.3508275 21.2133258 190 47.15767014 0.3264477 21.21186234 200 49.63609627 0.3042024 21.21039887 210 51.97065598 0.2842418 21.21186234 220 54.32701363 0.2649842 21.20893539 230 56.52642609 0.2477485 21.20893539 240 58.7432769 0.2310418 21.20308138 250 60.85331779 0.2157157 21.2074719 260 62.90886383 0.2012882 21.2074719 270 64.78348694 0.1885357 21.2074719 280 66.64503128 0.1762322 21.20893539 290 68.49349686 0.1643507 21.21039887 300 70.21117477 0.1535938 21.20893539 310 71.81768318 0.1437686 21.20893539 320 73.37187653 0.1344704 21.21039887 330 74.81272057 0.1260246 21.21039887 340 76.2949807 0.117504 21.21039887 350 77.5963179 0.1101589 21.21478926 360 78.84534004 0.103224 21.2162527 370 80.07038445 0.0965281 21.2162527 380 81.21913605 0.0903416 21.21917956 390 82.25235855 0.0848516 21.2162527 400 83.23326599 0.0797031 21.21771613 410 84.16621796 0.0748622 21.21917956 420 85.07955177 0.0701748 21.21917956 Average : 21.22103514 [CY+] In[CV+] W[CY+] Time (s]ransmittance (? Absorbance emperature (C) 30 11.9932283 0.921063899 22.49211652 40 12.45752449 0.90456825 21.68409753 50 12.94361862 0.887944292 21.60959388 60 13.4515107 0.871228939 21.58037082 70 14.08147125 0.851351967 21.55991267 80 14.67219551 0.833504895 21.54529869 90 15.28035812 0.815866467 21.5350684 100 15.88852074 0.798916535 21.52922232 110 16.55771759 0.780999529 21.51899137 120 17.2443528 0.763353101 21.50729833 130 17.97458389 0.745341155 21.4985282 140 18.80726532 0.725674349 21.49121951 150 19.55929436 0.708646817 21.48537241 160 20.36799805 0.691051655 21.48098699 170 21.20939865 0.673471645 21.47513964 180 22.0355407 0.656876289 21.46344453 190 23.03824608 0.637550587 21.45613481 200 23.95375969 0.620626312 21.45028687 210 24.93466713 0.603196425 21.4415147 220 25.85454033 0.587463179 21.43420432 230 26.87250427 0.570691859 21.42543157 240 27.95804184 0.55349325 21.42250725 250 28.95856743 0.538222926 21.41665851 260 29.99178993 0.522997614 21.40934738 270 31.02065285 0.508349066 21.40349832 280 32.14760651 0.492851356 21.40057374 290 33.27891975 0.47783078 21.3961868 300 34.30778267 0.46460735 21.39179978 310 35.37152229 0.451346249 21.38887505 320 36.55297081 0.43707732 21.38156308 330 37.66466591 0.42406588 21.38302549 340 38.81559731 0.410993726 21.37863823 350 39.87497734 0.399299551 21.3771758 360 41.03462791 0.3868495 21.37278843 370 42.12888466 0.375420038 21.36986347 380 43.20134346 0.364502747 21.36840098 390 44.30213959 0.353575299 21.36401346 400 45.57077988 0.341313539 21.35962585 410 46.62798012 0.331353397 21.35523816 420 47.76365295 0.320902466 21.35523816 430 48.82521278 0.311355855 21.35377558 440 49.94998665 0.301464624 21.34646254 450 50.94397285 0.29290719 21.34646254 460 51.97501556 0.284205372 21.34061195 470 52.97990074 0.27588886 21.33914928 480 54.06543831 0.267080272 21.3318358 490 55. 12045876 0.258687177 21.33476122 500 56.14060249 0.250722931 21.33329851 Avergae Temp 21.45178353 [CV+] In[CV+ 1/[CV+) Time (s) Transmittance (%) Absorbance Temperature (C) 30 15.35011154 0.81388846 29.19430928 40 17.15498123 0.76560975 34.86113697 50 19.12769508 0.71833736 35.0897507 60 21.17452194 0.67418639 34.97388801 70 23.39137276 0.63094429 34.77551282 80 25.64092049 0.59106639 34.58194108 90 27.97548019 0.55322245 34.37218527 100 30.26426422 0.51906988 34.20611164 110 32.65113899 0.48610166 34.02679888 120 35.01185623 0.45578486 33.86710576 130 37.42706833 0.42681419 33.71504171 140 39.75726845 0.40058346 33.5616487 150 42.13542404 0.37535263 33.409902 160 44.34791527 0.35312679 33.27167821 170 46.7108123 0.33058258 33.13951647 180 48.88842682 0.31079394 33.01636568 190 51.05078278 0.29199759 32.89479284 200 53.05401375 0.27528175 32.76442669 210 55.15315567 0.25842963 32.63564413 220 57.09099281 0.2434324 32.52026865 230 58.95253716 0.2294975 32.40201655 240 60.76394623 0.21635403 32.28679902 250 62.44020805 0.20453566 32.17608449 260 64.13608803 0.19289753 32.05806492 270 65.74041664 0.18216755 31.95338703 280 67.28589081 0.17207599 31.84287645 290 68.68313896 0.16314986 31.73979212 300 70.15450012 0.15394447 31.63234934 310 71.52995033 0.14551208 31.52055441 320 72.72447762 0.13831939 31.42499062 330 73.92554428 0.13120547 31.32653406 340 75.06993629 0.12453395 31.22665659 350 76.19253037 0.1180876 31.11948864 360 77.29550629 0.11184575 31.02851196 370 78.30911064 0.10618771 30.93170806 380 79.26168076 0.10093672 30.83201545 390 80.13577827 0.09617354 30.73969292 400 81.07962921 0.09108825 30.65180158 410 81.9253894 0.08658149 30.55662363 420 82.71229515 0.08242993 30.46441091 430 83.41636871 0.07874872 30.36930788 440 84.19237549 0.07472724 30.28301557 450 84.85721275 0.07131124 30.1865205 460 85.41088051 0.0684868 30.09736814 470 85.97544724 0.06562556 30.01701108 480 86.57271088 0.06261898 29.93813876 490 87.13073822 0.05982861 29.85490965 Average T: 32.11784374


 Kinetic Run 2
Kinetic Run 2







