Question
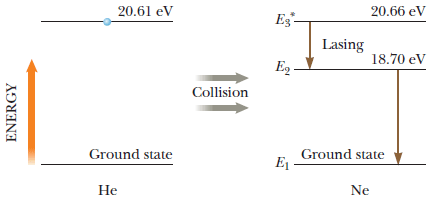
Neon has a metastable state, with an energy E 3 * = 20.66 eV above its ground state, that is useful for producing laser light.
Neon has a metastable state, with an energy E3* = 20.66 eV above its ground state, that is useful for producing laser light. Direct electric current passed through neon alone, however, will mostly excite lower energy levels only. Instead, in a helium-neon laser, electric current excites the helium atoms to an energy 20.61 eV above that element's ground state. By a fortunate coincidence, this energy nearly matches that of the E3* level in neon. So, when an excited helium atom collides with a neon atom, there is enough energy (along with a small amount of kinetic energy from the atoms) to excite the neon atom to the E3* energy level. Lasing action then takes place for transitions from E3* to E2 in the neon atoms.From the data in the figure below, calculate the wavelength (in nm) of laser light emitted by the helium-neon laser.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


