Question
neutrons were given by the following symbol: n.An alpha particle (a helium atom) is designated as He or a 239 The collision of Pu

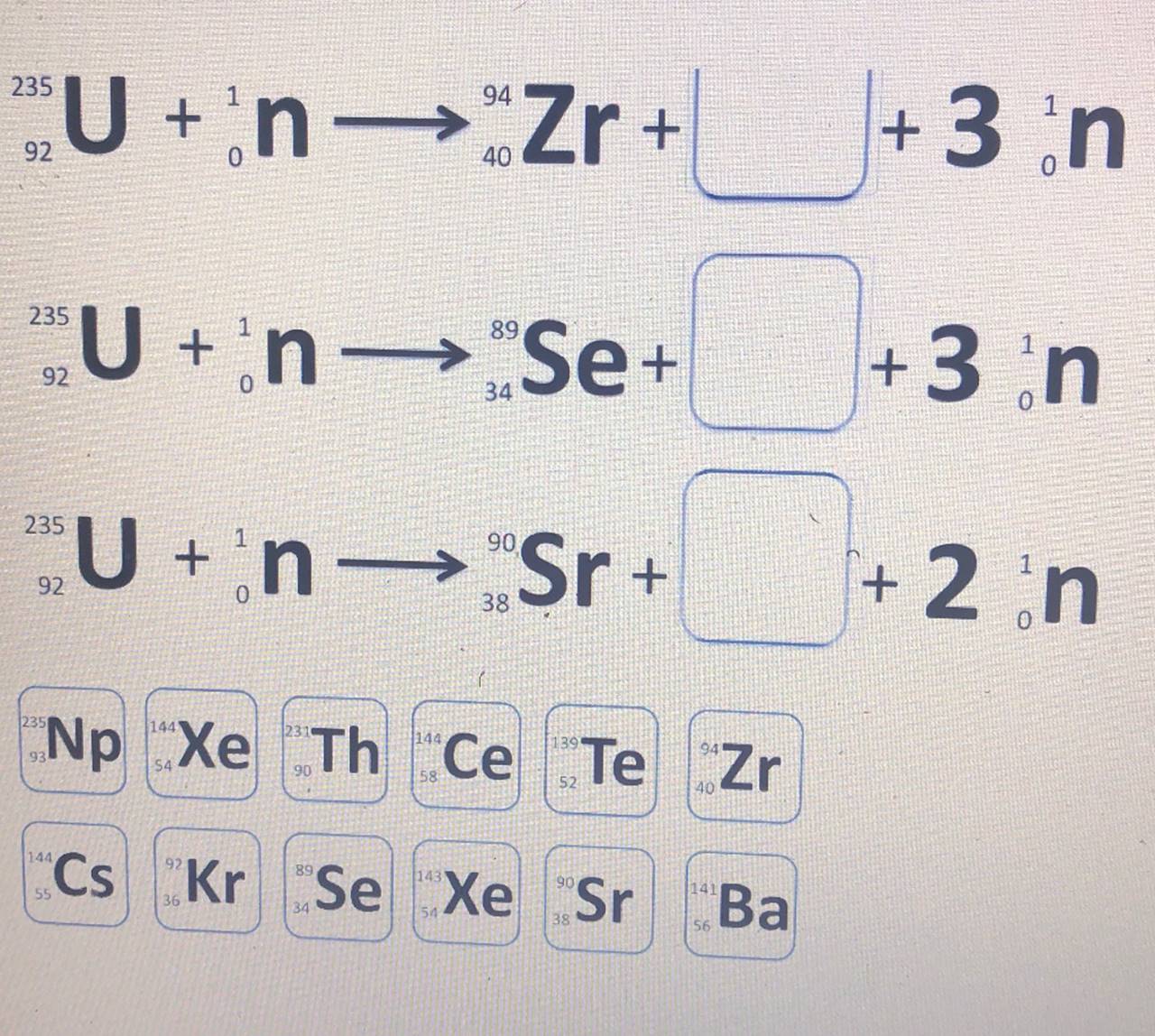
neutrons were given by the following symbol: n.An alpha particle (a helium atom) is designated as He or a 239 The collision of Pu with an alpha particle generates a new isotope and one neutron. What is the new 94 isotope that is produced in this nuclear reaction? U +n Zr+ 235 +3n 94 92 40 235 U +n "Se+ 89 +3 n 92 34 235 U +n Sr+ 90 +2 n 92 38 Np CeTe Zr 235 144 XeTh 144 90 52 40 Cs Kr Se Xe Sr 144 89 143 90 34 38
Step by Step Solution
3.53 Rating (160 Votes )
There are 3 Steps involved in it
Step: 1
An element can be represented by using the notation X Here X is ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Intermediate Microeconomics and Its Application
Authors: walter nicholson, christopher snyder
11th edition
9781111784300, 324599102, 1111784302, 978-0324599107
Students also viewed these Accounting questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



