Answered step by step
Verified Expert Solution
Question
1 Approved Answer
NEWS WIRE: R&D BARRIERS Stifling Would-Be Competition Washington, DC-The Federal Drug Administration (FDA) today named and shamed the biggest pharmaceutical companies for stifling the
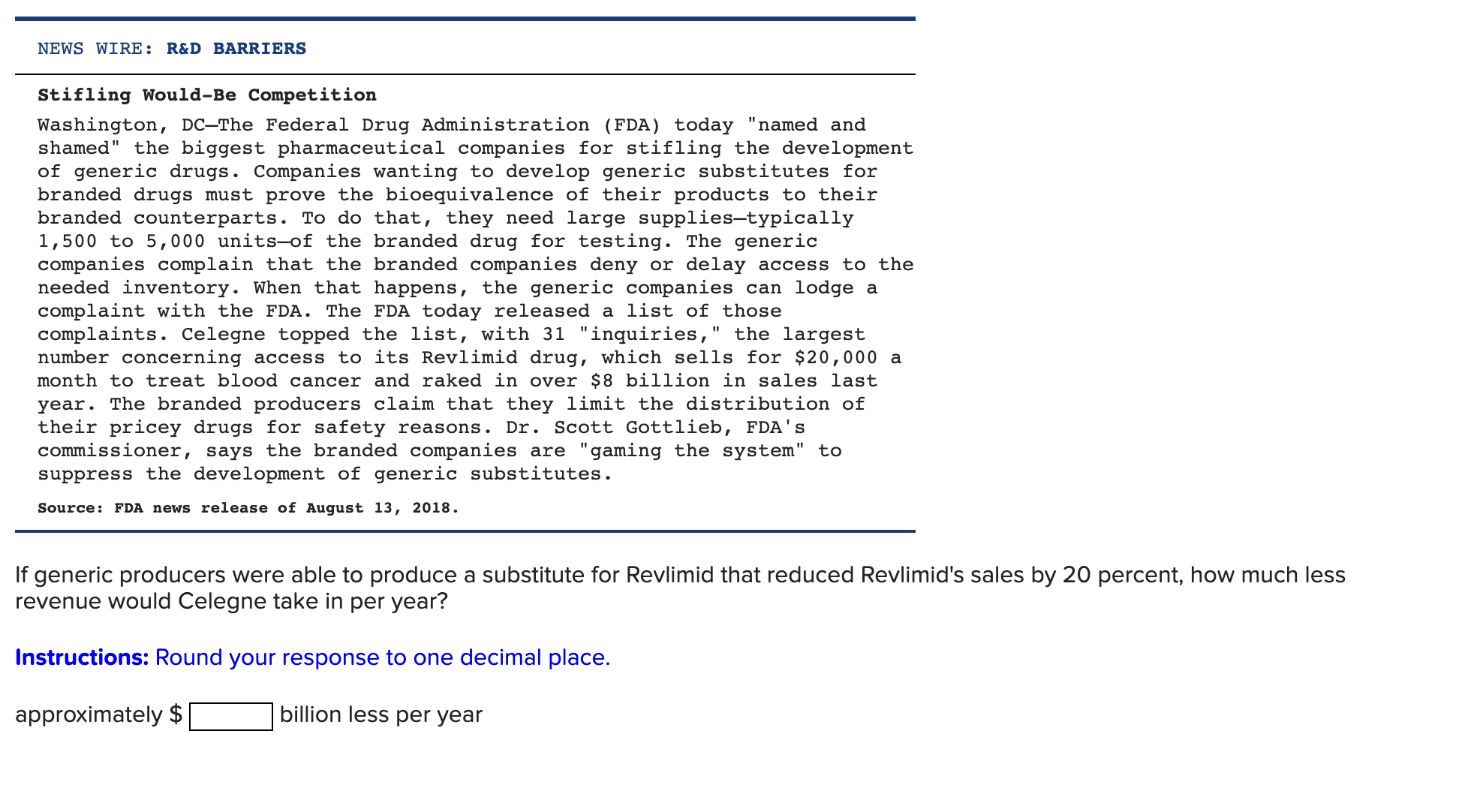
NEWS WIRE: R&D BARRIERS Stifling Would-Be Competition Washington, DC-The Federal Drug Administration (FDA) today named and shamed" the biggest pharmaceutical companies for stifling the development of generic drugs. Companies wanting to develop generic substitutes for branded drugs must prove the bioequivalence of their products to their branded counterparts. To do that, they need large supplies-typically 1,500 to 5,000 units-of the branded drug for testing. The generic companies complain that the branded companies deny or delay access to the needed inventory. When that happens, the generic companies can lodge a complaint with the FDA. The FDA today released a list of those complaints. Celegne topped the list, with 31 "inquiries," the largest number concerning access to its Revlimid drug, which sells for $20,000 a month to treat blood cancer and raked in over $8 billion in sales last year. The branded producers claim that they limit the distribution of their pricey drugs for safety reasons. Dr. Scott Gottlieb, FDA's commissioner, says the branded companies are "gaming the system" to suppress the development of generic substitutes. Source: FDA news release of August 13, 2018. If generic producers were able to produce a substitute for Revlimid that reduced Revlimid's sales by 20 percent, how much less revenue would Celegne take in per year? Instructions: Round your response to one decimal place. approximately $ billion less per year
Step by Step Solution
There are 3 Steps involved in it
Step: 1
Answer To calculate the reduction in revenue for Celeg...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


