Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Nickel (11) ions react quantitatively with dimethylglyoxime (C4H8O2N2) forming a complex which precipitates out as a red solid. The equation for the reaction and the
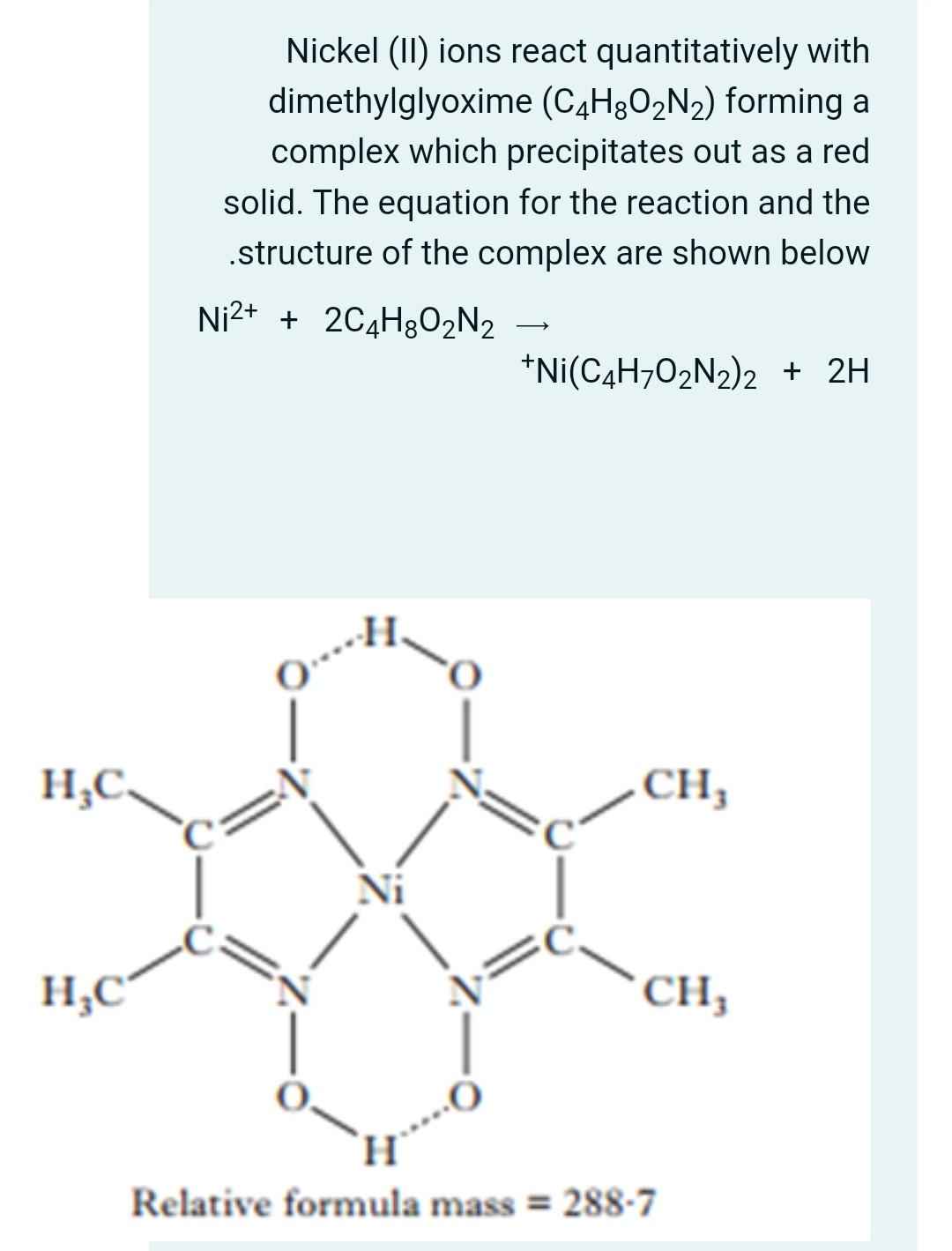
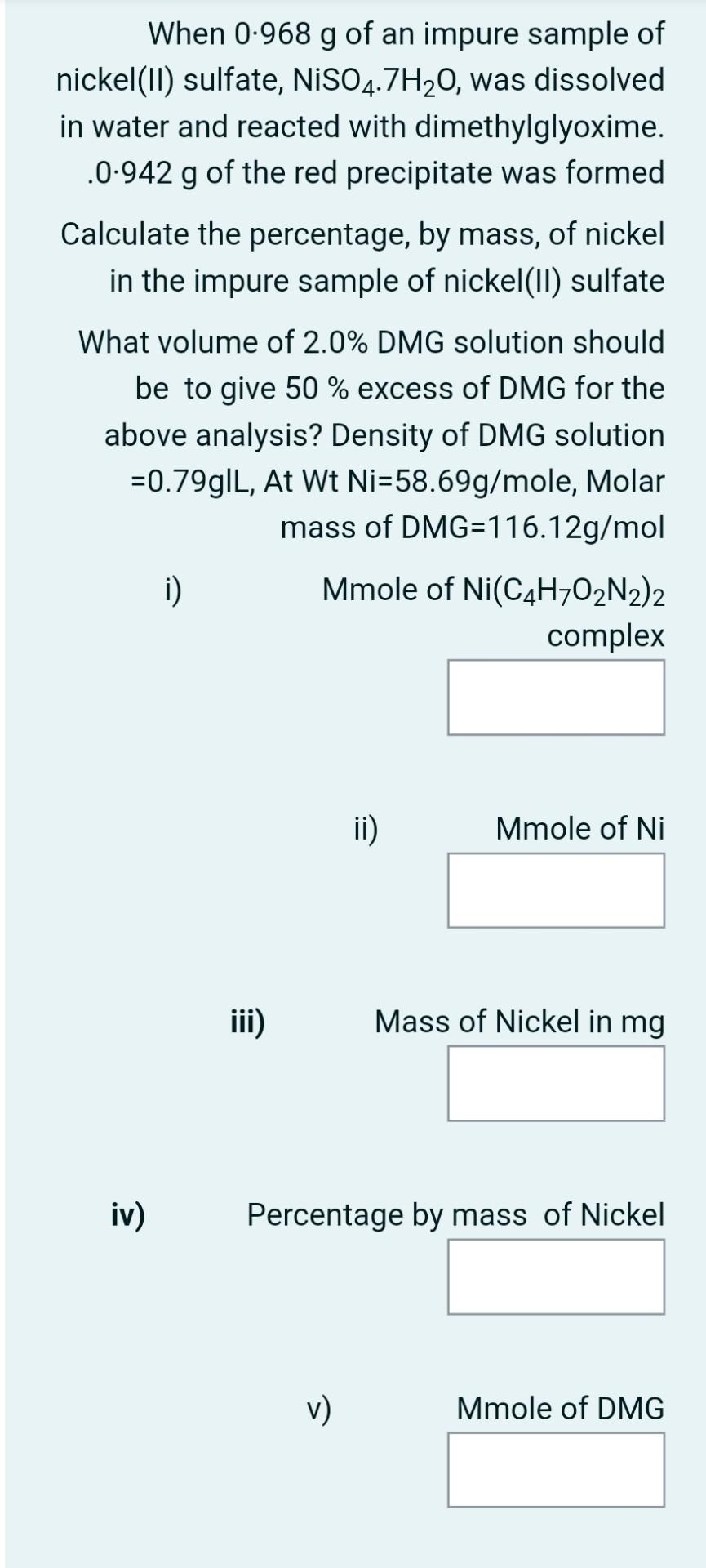

Nickel (11) ions react quantitatively with dimethylglyoxime (C4H8O2N2) forming a complex which precipitates out as a red solid. The equation for the reaction and the structure of the complex are shown below Ni2+ + 2C4H8O2N2 *Ni(C4H702N2)2 + 2H HC CH; Ni HC CH; H Relative formula mass = 288.7 When 0.968 g of an impure sample of nickel(II) sulfate, NiSO4.7H20, was dissolved in water and reacted with dimethylglyoxime. .0.942 g of the red precipitate was formed Calculate the percentage, by mass, of nickel in the impure sample of nickel(II) sulfate What volume of 2.0% DMG solution should be to give 50 % excess of DMG for the above analysis? Density of DMG solution =0.79g|L, At Wt Ni=58.69g/mole, Molar mass of DMG=116.12g/mol i) Mmole of Ni(C4H7O2N2)2 complex ii) Mmole of Ni iii) Mass of Nickel in mg iv) Percentage by mass of Nickel v) Mmole of DMG vi) Mass of DMG in mg vii) Mass of DMG in grams to give 50 excess of DMG solution viii) Mass of DMG in grams to give 50 excess of 2%DMG solution ix) Volume of 2% DMG solution
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


