Answered step by step
Verified Expert Solution
Question
1 Approved Answer
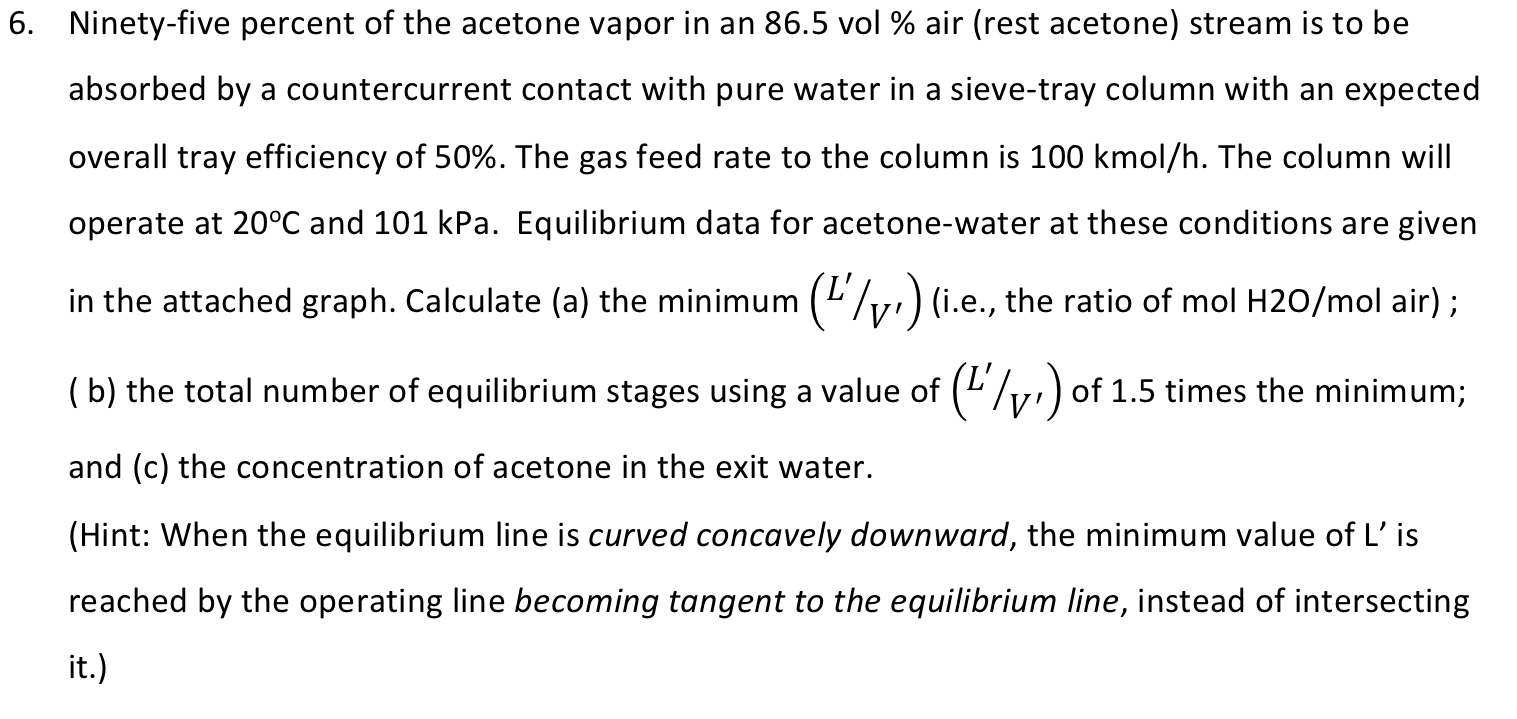
Ninety - five percent of the acetone vapor in an 8 6 . 5 vol % air ( rest acetone ) stream is to be
Ninetyfive percent of the acetone vapor in an vol air rest acetone stream is to be
absorbed by a countercurrent contact with pure water in a sievetray column with an expected
overall tray efficiency of The gas feed rate to the column is kmo The column will
operate at and kPa. Equilibrium data for acetonewater at these conditions are given
in the attached graph. Calculate a the minimum ie the ratio of molH air ;
b the total number of equilibrium stages using a value of of times the minimum;
and c the concentration of acetone in the exit water.
Hint: When the equilibrium line is curved concavely downward, the minimum value of is
reached by the operating line becoming tangent to the equilibrium line, instead of intersecting
it
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


