Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Nitric acid is commercially prepared from nitric oxide, which is produced by oxidation of ammonia in the gas phase: The feed consists of 15 mol
Nitric acid is commercially prepared from nitric oxide, which is produced by oxidation of ammonia in the gas phase:
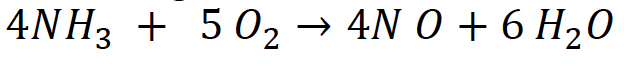
The feed consists of 15 mol of ammonia with air at 8.2 atm and 227 c. a) calculate the total concentration at the entrance b) What concentration is the ammonia in the inlet? c) prepare a stoichiometric table using ammonia as the calculation basis, for a constant pressure and isothermal batch system. Find i. Later Express the C of each of the species as a function of the X. Find i. d) Prepare a stoichiometric table using ammonia as the basis of calculation, for a constant pressure and isothermal flow system. Then express the C of each one of the species as a function of X. Find theta i, epsilon ,4NH3+5O24NO+6H2O
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


