Answered step by step
Verified Expert Solution
Question
1 Approved Answer
no 13 of 29.0.) 3.13. A liquid mixture is prepared by combining N different liquids with densities 1,2N. The volume of component i added to
no 13 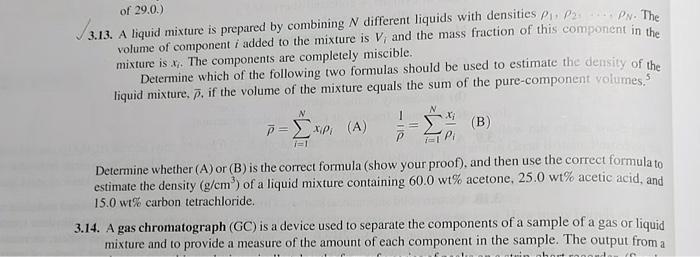
of 29.0.) 3.13. A liquid mixture is prepared by combining N different liquids with densities 1,2N. The volume of component i added to the mixture is Vi and the mass fraction of this component in the mixture is xi. The components are completely miscible. Determine which of the following two formulas should be used to estimate the density of the liquid mixture, , if the volume of the mixture equals the sum of the pure-component yolumes, 5 =i=1Nxii(A)1=i=1Nixi Determine whether (A) or (B) is the correct formula (show your proof), and then use the correct formula to estimate the density (g/cm3) of a liquid mixture containing 60.0wt% acetone, 25.0wt% acetic acid, and 15.0 w\% carbon tetrachloride. 3.14. A gas chromatograph (GC) is a device used to separate the components of a sample of a gas or liquid mixture and to provide a measure of the amount of each component in the sample. The output from a 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


