Answered step by step
Verified Expert Solution
Question
1 Approved Answer
no need for solutions just the answers please ASAP A three-halves order reaction has rate law -r=kC32. The linearized form of the rate about Co
no need for solutions just the answers please ASAP 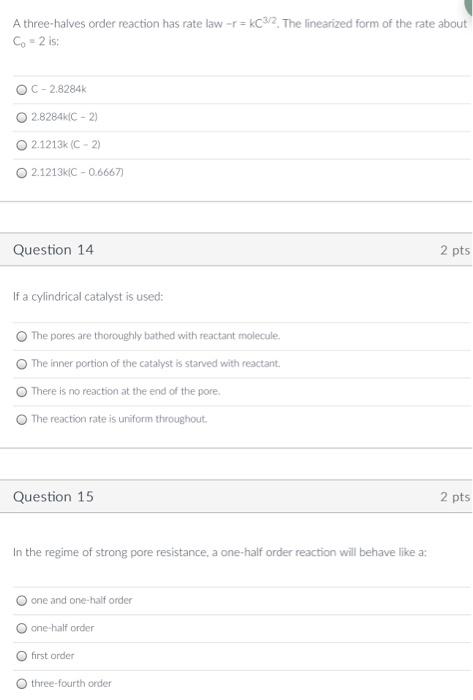
A three-halves order reaction has rate law -r=kC32. The linearized form of the rate about Co - 2 is: OC - 2.8284 2.8284C-2) 21213k (C - 2) 02.1213KC -0.6667) Question 14 2 pts If a cylindrical catalyst is used: The pores are thoroughly bathed with reactant molecule, The inner portion of the catalyst is starved with reactant There is no reaction at the end of the pore. The reaction rate is uniform throughout Question 15 2 pts In the regime of strong pore resistance, a one-half order reaction will behave like a: o one and one-half order one-half order o first order three-fourth order 
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


