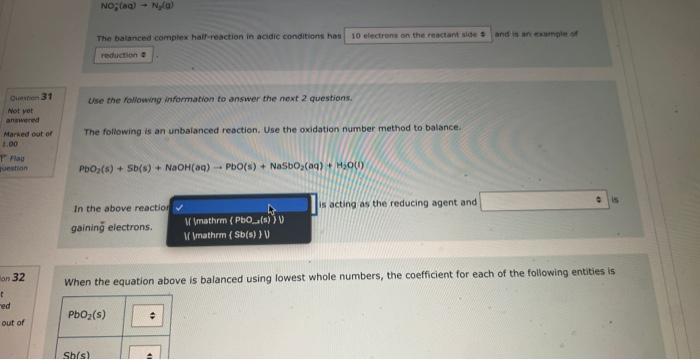
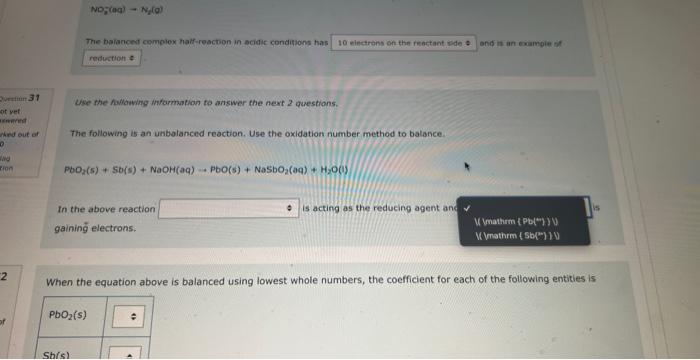
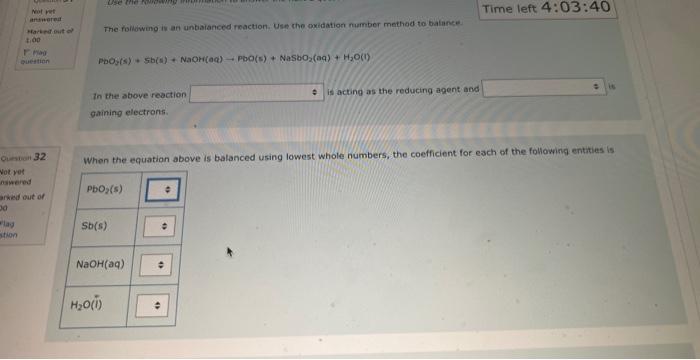
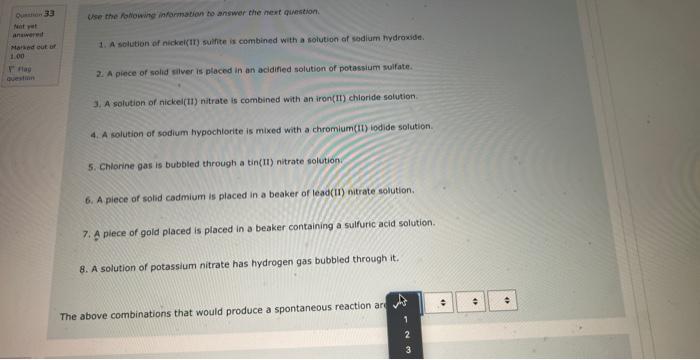
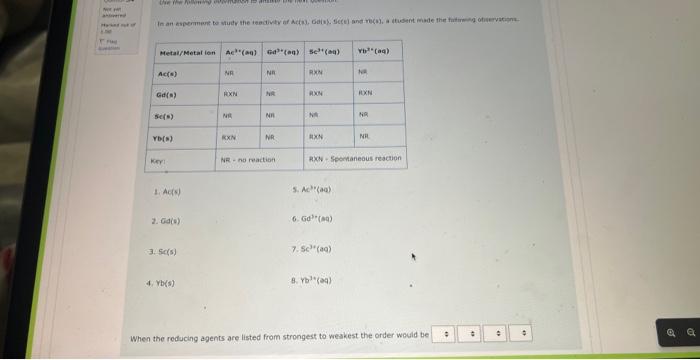
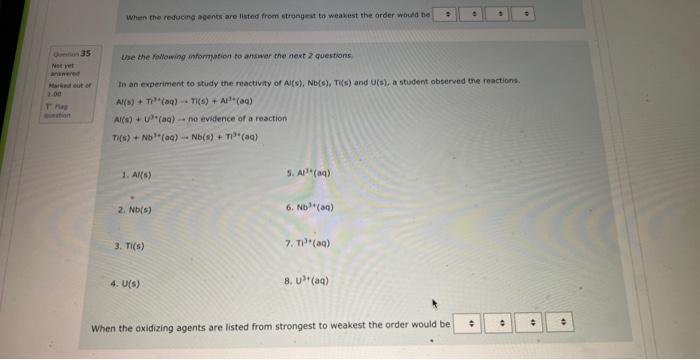
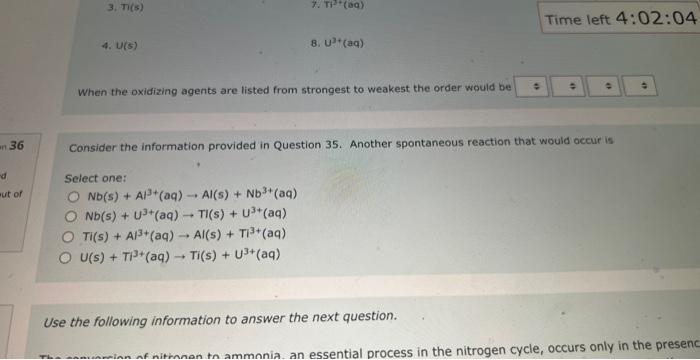
NOg(aa)Ny(a) The balanced complex haif-reaction in acidic conditions has and in an eamangien of Ouevinen 31 Use the following information to answer the noxt 2 questians. Not vot anzwered Marked out of The following is an unbalanced reoction. Use the oxidation number method to balance. 4.00 PbO2(s)+5b(s)+NaOH(aq)PbO(s)+NaSbO2(aq)+H2O(l) In the above reactio is acting as the reducing agent and gainin electrons. When the equation above is balanced using lowest whole numbers, the coefficient for each of the following entities is out of The balanced complox hatf-reaction in acidic conditions has and n an eximpie of Wie the holawing infarmarion to answar the next 2 questions. The following is an unbolanced reaction. Use the oxidation number method to balance. PbO2(5)+Sb(s)+NaOH(aq)=PbO(5)+NaSbO2(aq)+H2O(s) In the above reaction is actifg as the reducing agent and gaining electrons. When the equation above is balanced using lowest whole numbers, the coefficient for each of the following entities is The following is an unbaranced reaction. Use the oxidation rumber methed to balance. PbOS(s)+Sb(s)+NaOH(aq)PbO(s)+NaSbO2(aq)+H2O(1) in the above reaction gaining electronti. Qurawin 32 When the equation above is balanced using lowest whole numbers, the coefficient for each of the following entries is Not yer nswered arked out of Wese the fonowwe infarmation to answer the next quhestion. 1. A soliztion of nickei(tr) suinte is combined with a solution af sodium hydraxide. 2. A pince of solid silver is placced in an acidified solution of potassium sulfate. 3. A solution of nickel(I1) nitrate is combined with an iron(II) chloride solution. 4. A solution of sodium hypochlorite is mixed with a chromium(ti) todide solution. 5. Chlarine gas is bubbled through a tin(II) nitrate solution. 6. A piece of solid cadmium is placed in a beaker of lead(II) nitrate solution. 7. A piece of gold placed is placed in a beaker containing a sulfuric acid solution. 8. A solution of potassium nitrate has hydrogen gas bubbled through it. When the reducing agents are listed from strongest to weakest the order would be When che reducing agents are listed from ktrungest ta weavest the order waud be Wye the following infomafion fo answar the flest 2 questions: In an evperimient to study the reactivity of Al(s), Nb(s), Ti(s) and u(s), a student observed the reactions. A(s)+Ti3+(aq)=Ti(s)+AL3+(ac)A(s)+U2+(aq)=NeevidenceofareactionT(s)+Nbb3+(aq)=Nb(s)+T3+(aq) d. A ({s) 5. AI3+(aq) 2. No(s) 6. (Ab3+(at) 3. Ti(s) 7. Ti3(aq) 4. U(s) 8. u2+(aq) When the oxidizing agents are listed from strongest to weakest the order would be 4. u(5) 8. u3+(aq) When the oxidizing agents are listed from strongest to weakest the order would be Consider the information provided in Question 35. Another spontaneous reaction that would occur is Select one: Nb(s)+Al3+(aq)Al(s)+Nb3+(aq)Nb(s)+U3+(aq)Tl(s)+U3+(aq)Ti(s)+Al3+(aq)Al(s)+Tl3+(aq)U(s)+Ti3+(aq)Ti(s)+U3+(aq) Ise the following information to answer the next