Question: nolpe2 Procedure Step 1: Shake the cubes in a container, and roll them onto a flat surface. When rolled, each die can come up
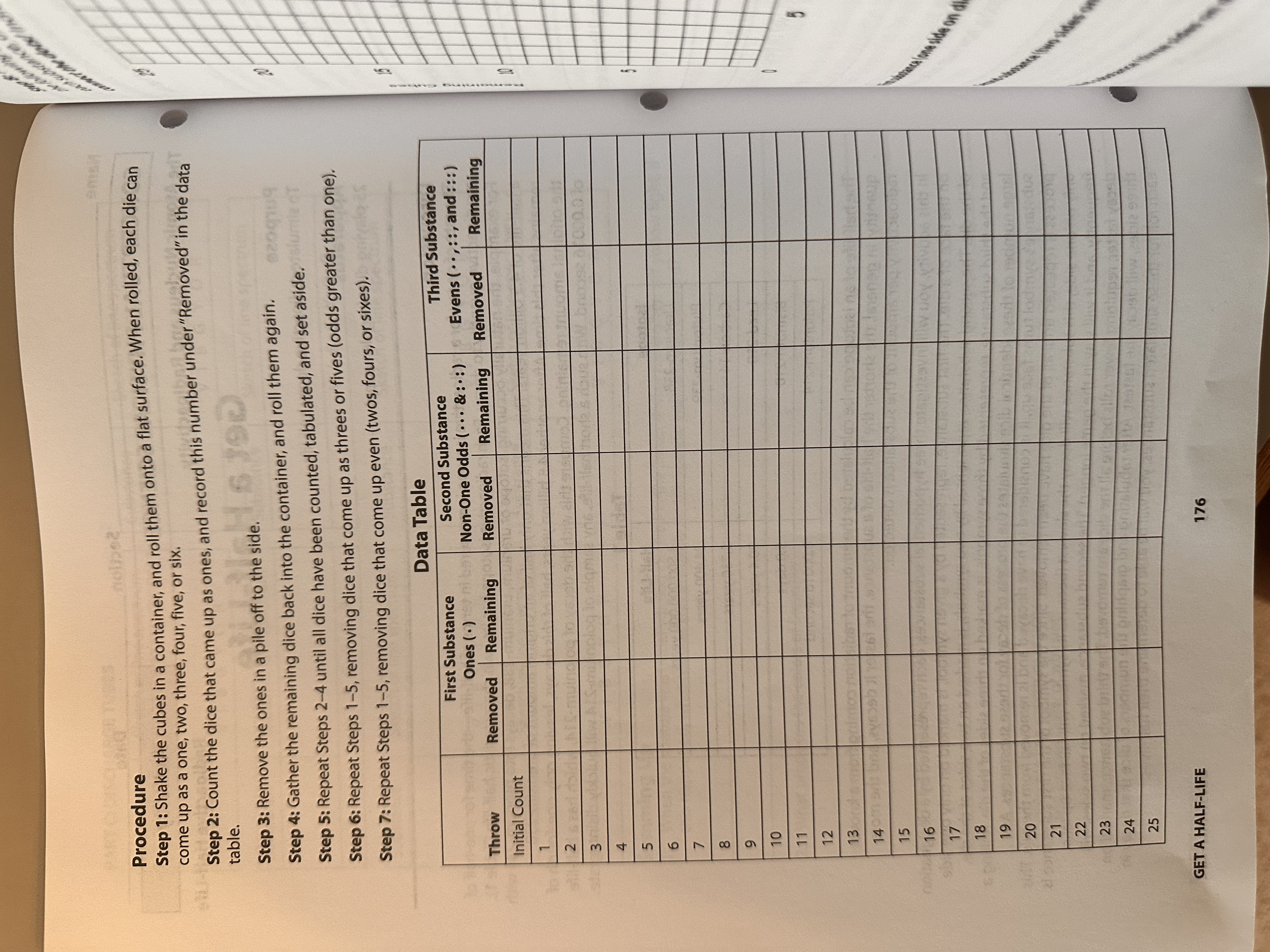


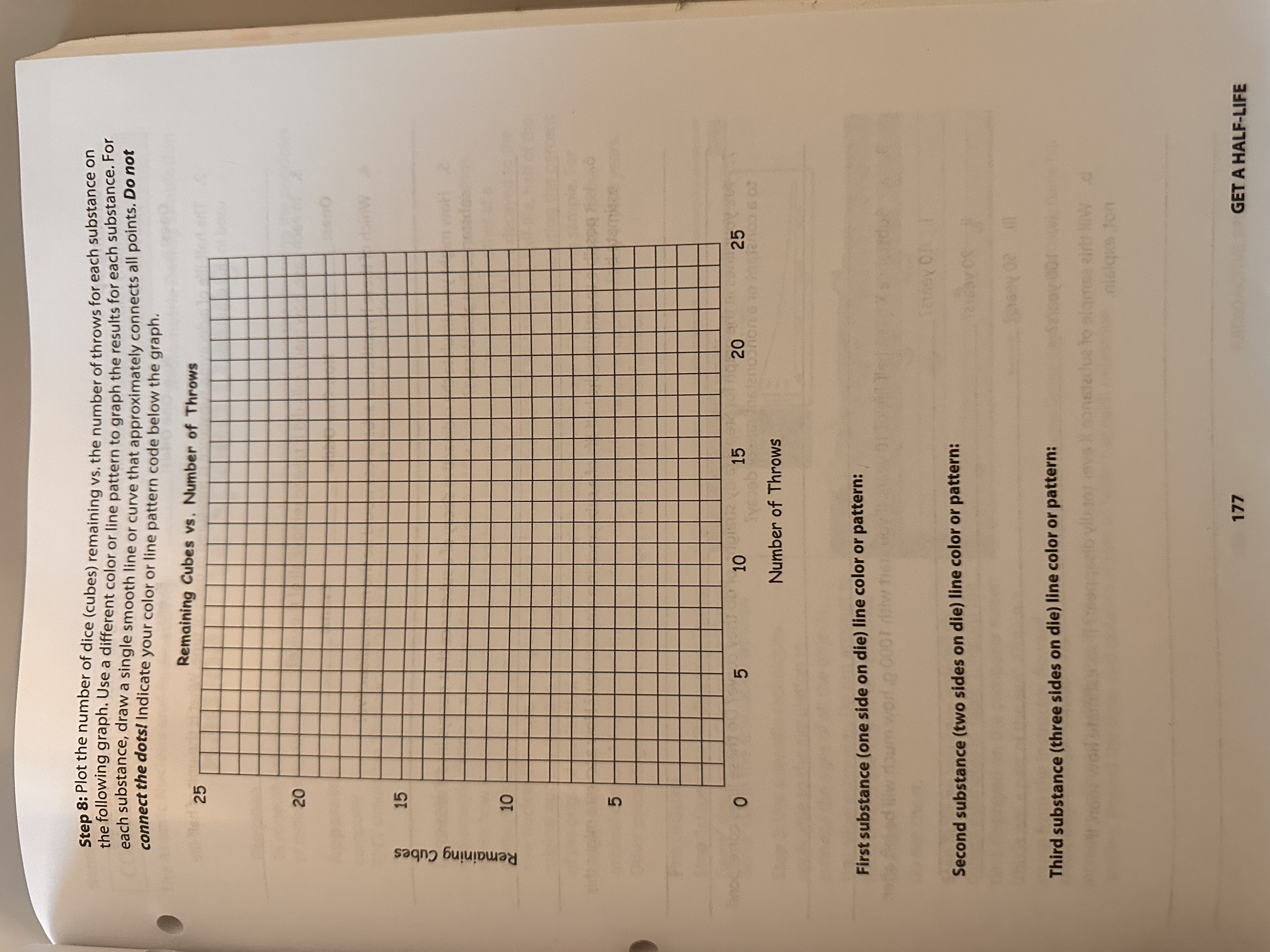
nolpe2 Procedure Step 1: Shake the cubes in a container, and roll them onto a flat surface. When rolled, each die can come up as a one, two, three, four, five, or six. Step 2: Count the dice that came up as ones, and record this number under "Removed" in the data table. f to the side. 5 190 Step 3: Remove the ones in a pile off to the side. Step 4: Gather the remaining dice back into the container, and roll them again. Step 5: Repeat Steps 2-4 until all dice have been counted, tabulated, and set aside. Step 6: Repeat Steps 1-5, removing dice that come up as threes or fives (odds greater than one). Step 7: Repeat Steps 1-5, removing dice that come up even (twos, fours, or sixes). Data Table Throw First Substance Ones () Removed Remaining Second Substance Non-One Odds (...&::) Removed Remaining Third Substance Evens (,::, and :::) Removed Remaining Initial Count 1 2 3 4 5 6 7 8 9 10 11 12 13 11261 14 15 16 17 18 19 20 21 22 23 24 25 25 GET A HALF-LIFE 176 00 5 tance lone side on di no Summing Up 101 jo) salb 1. How many rolls did it take for the number of each dice set to be reduced by half? These are your half-life readings. Ones: Non-One Odds: Evens: 2. The half-life of a decaying substance is measured in units of time. What is the unit of half-life used in this simulation? 3. In each case, how many rolls did it take to remove all of the dice? Ones: Non-One Odds: Evens: 4. Which of these hypothetical substances would be the most radioactive? Name CO The A 5. How might you simulate the radioactive decay of a substance that decays into a second substance that also decays? F H E J C P T A C P 6. Is it possible to estimate the half-life of a substance in a single throw? How accurate might this estimate be? 7. Are your lines in the graph for Step 8 fairly straight, or do they curve? Do these lines correspond to a constant or a nonconstant rate of decay? 8. a. Substance X has a half-life of 10 years. If you start with 1000 g, how much will be left after: i. 10 years? ii. 20 years? iii. 50 years? iv. 100 years? not, explain. b. Will this sample of substance X ever totally disappear? If so, estimate how soon. If C F G G S a e th ha SC D St CO tin (th sta Cre in GET A HALF-LIFE 178 m light. If agen Name Section Date CONCEPTUAL PHYSICAL SCIENCE The Atomic Nucleus and Radioactivity Purpose Get a Half-Life To simulate radioactive decay half-life Apparatus 25 playing dice (regular, cube) Discussion Activity Radioactive Half-Life The rate of decay for a radioactive isotope is measured in terms of half-life-the time for one-half of a radioactive quantity to decay. Each radioactive isotope has its own characteristic half-life (Table 1). For example, the naturally occurring isotope of uranium, uranium-238, decays into thorium-234 with a half-life of 4.5 billion years. This means that only half of an original amount of uranium-238 remains after this time. After another 4.5 billion years, half of this decays, leaving only one-fourth of the original amount remaining. Compare this with the decay of polonium-214, which has a half-life of 0.00016 second. With such a short half-life, any sample of polonium-214 will quickly disintegrate. Table 1 Isotope Uranium-238 Half-Life 4,500,000,000 years Plutonium-239 24,400 years Carbon-14 5,730 years Lead-210 Bismuth-210 20.4 years 5.0 days Polonium-214 0.00016 second The half-life of an isotope can be calculated by the amount of radiation coming from a known quantity. In general, the shorter the half-life of a substance, the faster it decays, and the more radioactivity per amount of the substance is detected. In this activity, you will investigate three hypothetical substances, each represented by a designation on the face of a die. The first substance, represented by a given symbol, is marked on only one side of the die. The second substance, represented by two symbols, is marked on two sides of the die, and the third substance, represented by three symbols, is marked on three sides of the die. Rolling a large number of these identical dice simulates the process of decay for these substances. As a substance's symbol turns face up, it is considered to have decayed and is removed from the pile. This process is repeated until all of the dice have been removed. Since the symbol of the first substance is only on one side, this substance will decay the slowest (because its symbol will fall face up least frequently, and it will stay in the game longer). The second substance, marked on two sides, will decay faster, requiring fewer rolls before all the dice are removed. The third substance, marked on three sides, will decay the fastest. After tabulating and graphing the numbers of dice that decay in each roll for these simulated substances, you will be able to determine their half-lives. 175 GET A HALF-LIFE Step 8: Plot the number of dice (cubes) remaining vs. the number of throws for each substance on the following graph. Use a different color or line pattern to graph the results for each substance. For each substance, draw a single smooth line or curve that approximately connects all points. Do not connect the dots! Indicate your color or line pattern code below the graph. Remaining Cubes vs. Number of Throws Remaining Cubes 25 25 20 15 10 10 5 W 5 10 15 20 Insenconion 20 25 Number of Throws First substance (one side on die) line color or pattern: doum worl. 0007 w te Second substance (two sides on die) line color or pattern: Third substance (three sides on die) line color or pattern: uz to ala 177 02.1 nisqus ton GET A HALF-LIFE
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


