Answered step by step
Verified Expert Solution
Question
1 Approved Answer
not sure what other information I can give thats all I have. I need to fill out the table for part c and draw 2

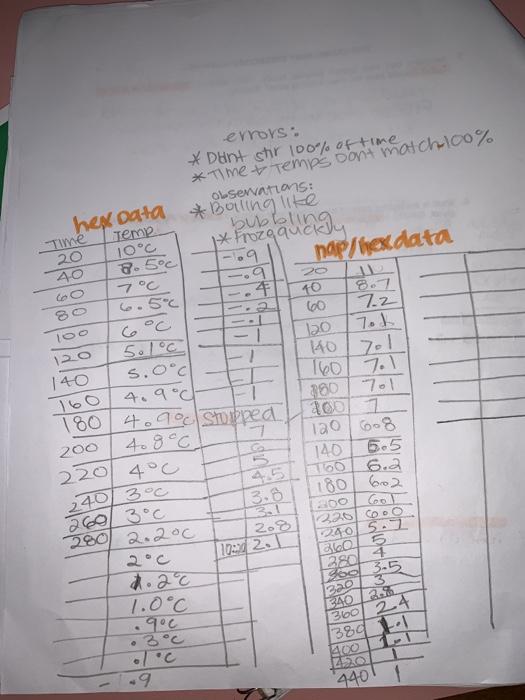
not sure what other information I can give thats all I have. I need to fill out the table for part c and draw 2 cooling curves. 1 for naphthalene and cyclohexane and 1 for just cyclohexane. I provided data from the experiment to do so I know my data isnt 100% accurate but please try to help me make these 2 cooling curve images
I need help making the cooling curve for part c and not sure what to do I tried getting help from someone on chegg 3+ times already
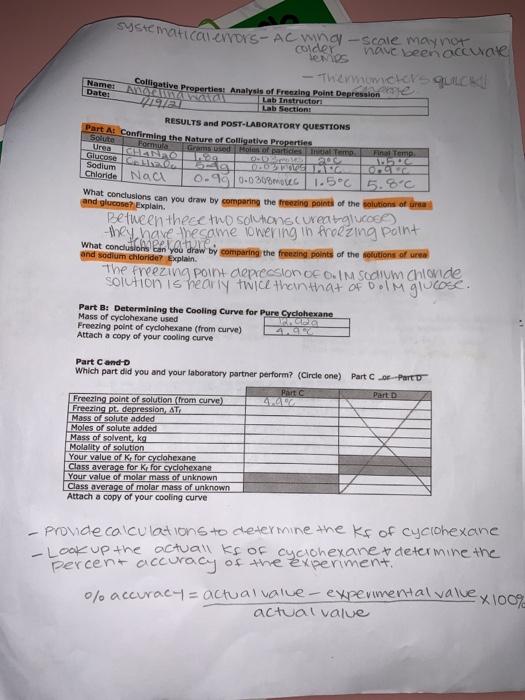
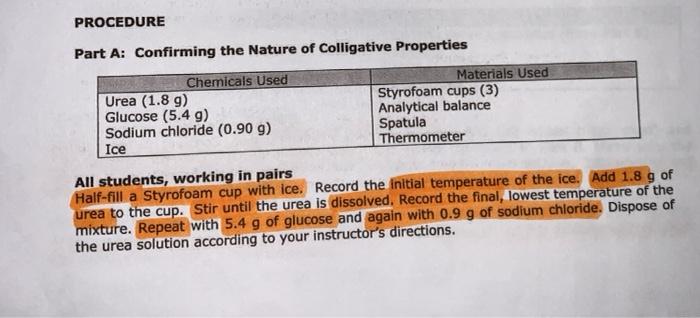
soient is cyclohexane for part Bro. Part B: Determining the Cooling Curve for Pure Cyclohexane Chemicals Used Cyclohexane Naa Ice Materials Used 200-mm test tube 400-ml beaker 600-ml beaker 20-ml graduated cylinder Thermometer Ring stand Test tube clamp Stopwatch All students, working in pairs 140.729 1. Place a clean, dry 200-mm test tube in a 400-ml. benker Record the mass. Using the 19914 or cyclohexane. Pour this volume into the test tube. Reweigh the test tube, the beaker and the cyclohexane. Wrap a paper towel around the 400-ml beaker and place inside 2.925 -Fill with 2 grams of NaCl to the ice 2. Place the 600-ml beaker containing the ice bath and the test tube on a ring stand. Attach a test tube clamp to the ring stand to hold the test tube. lower the thermometer completely into the test tube filled with cyclohexane. 3. While constantly stirring the cyclohexane with a thermometer, record the temperature (+0.2C) at 20 second intervals. Once the cyclohexane solidifies the temperature remains virtually constant. Continue collecting data at 20 second Intervals until the temperature begins to drop again (typically after a total of 10 minutes). 4. Allow the cyclohexane to melt before reusing in Part C or D. Part C: Determining the Kfor Cyclohexane Chemicals Used Materials Used Naphthalene (CH) Cooling curve apparatus (from Part B) Naci Spatula Ice Analytical balance Stopwatch Half the class, working in pairs Measure approximately 0.30 grams of naphthalene (record the actual mass used) and transfer into the test tube filled with cyclohexane that you used in Part B. Make sure the naphthalene is completely dissolved before proceeding to the next section. Add Ice and salt to the 400-ml beaker as needed. Determine the freezing point of the solution in the same way you did for the pure solvent Again, be sure to collect data for at least 10 minutes. Dispose of the cyclohexane solution according to your instructor's directions IV) Time DV) Temperatureloc) * Sotton Of Napolein in & Freezing cyclovexaine decreasing the temperature. Time * Choge of time AT=mok Morality moles soute ene Rojavich (sold those) Freezing PC depresor concc Adezing Temperame Tf- Tf kg solvent Jubbolingu Time 20 40 Coo 80 errors: * Dent stir 100% of time *Time & Temps Don't match 100% Observations: hexdata *Bolling like Temp. 10C 8.5C nap/hex.data 7 7c 9 L 4 6.5C 10 6oc 100 7.2 120 5.1C 120 7.1 140 s.0c - 4.900 180 4.90 Stopped 380 200 408c 120 608 220 4C 140 6.5 100 240/300 180 602 3.8 260 3C 300 280 2.200 208 220 CDO 240 5.1 Blo 3804 Foo 3.5 140 1601 7.1 7.1 160 6.2 102421 zad 1.0C .900 BAD AS . 360 24 380 9 440 soient is cyclohexane for part Bro. Part B: Determining the Cooling Curve for Pure Cyclohexane Chemicals Used Cyclohexane Naa Ice Materials Used 200-mm test tube 400-ml beaker 600-ml beaker 20-ml graduated cylinder Thermometer Ring stand Test tube clamp Stopwatch All students, working in pairs 140.729 1. Place a clean, dry 200-mm test tube in a 400-ml. benker Record the mass. Using the 19914 or cyclohexane. Pour this volume into the test tube. Reweigh the test tube, the beaker and the cyclohexane. Wrap a paper towel around the 400-ml beaker and place inside 2.925 -Fill with 2 grams of NaCl to the ice 2. Place the 600-ml beaker containing the ice bath and the test tube on a ring stand. Attach a test tube clamp to the ring stand to hold the test tube. lower the thermometer completely into the test tube filled with cyclohexane. 3. While constantly stirring the cyclohexane with a thermometer, record the temperature (+0.2C) at 20 second intervals. Once the cyclohexane solidifies the temperature remains virtually constant. Continue collecting data at 20 second Intervals until the temperature begins to drop again (typically after a total of 10 minutes). 4. Allow the cyclohexane to melt before reusing in Part C or D. Part C: Determining the Kfor Cyclohexane Chemicals Used Materials Used Naphthalene (CH) Cooling curve apparatus (from Part B) Naci Spatula Ice Analytical balance Stopwatch Half the class, working in pairs Measure approximately 0.30 grams of naphthalene (record the actual mass used) and transfer into the test tube filled with cyclohexane that you used in Part B. Make sure the naphthalene is completely dissolved before proceeding to the next section. Add Ice and salt to the 400-ml beaker as needed. Determine the freezing point of the solution in the same way you did for the pure solvent Again, be sure to collect data for at least 10 minutes. Dispose of the cyclohexane solution according to your instructor's directions IV) Time DV) Temperatureloc) * Sotton Of Napolein in & Freezing cyclovexaine decreasing the temperature. Time * Choge of time AT=mok Morality moles soute ene Rojavich (sold those) Freezing PC depresor concc Adezing Temperame Tf- Tf kg solvent Jubbolingu Time 20 40 Coo 80 errors: * Dent stir 100% of time *Time & Temps Don't match 100% Observations: hexdata *Bolling like Temp. 10C 8.5C nap/hex.data 7 7c 9 L 4 6.5C 10 6oc 100 7.2 120 5.1C 120 7.1 140 s.0c - 4.900 180 4.90 Stopped 380 200 408c 120 608 220 4C 140 6.5 100 240/300 180 602 3.8 260 3C 300 280 2.200 208 220 CDO 240 5.1 Blo 3804 Foo 3.5 140 1601 7.1 7.1 160 6.2 102421 zad 1.0C .900 BAD AS . 360 24 380 9 440 Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


