Answered step by step
Verified Expert Solution
Question
1 Approved Answer
6-30 Carbon dioxide at an initial pressure of 100 atm and a temperature of 300 K under- goes an adiabatic free expansion in which

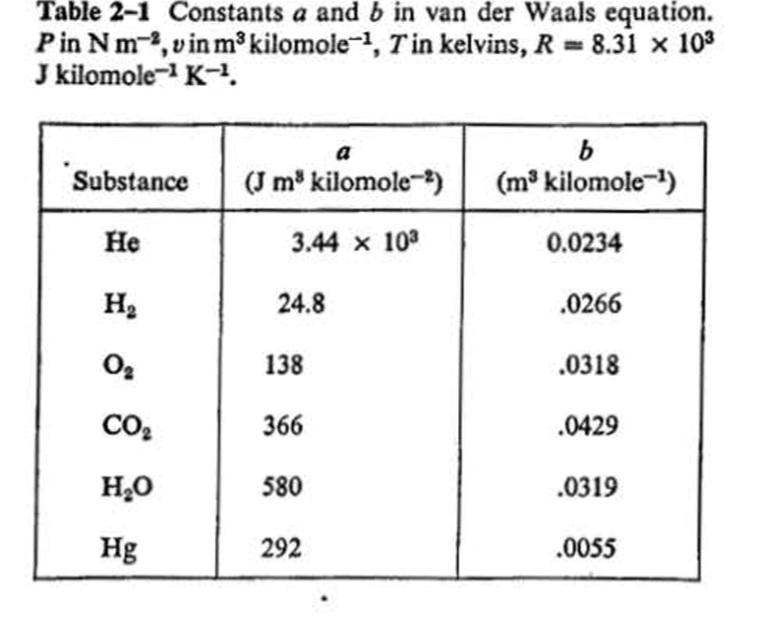
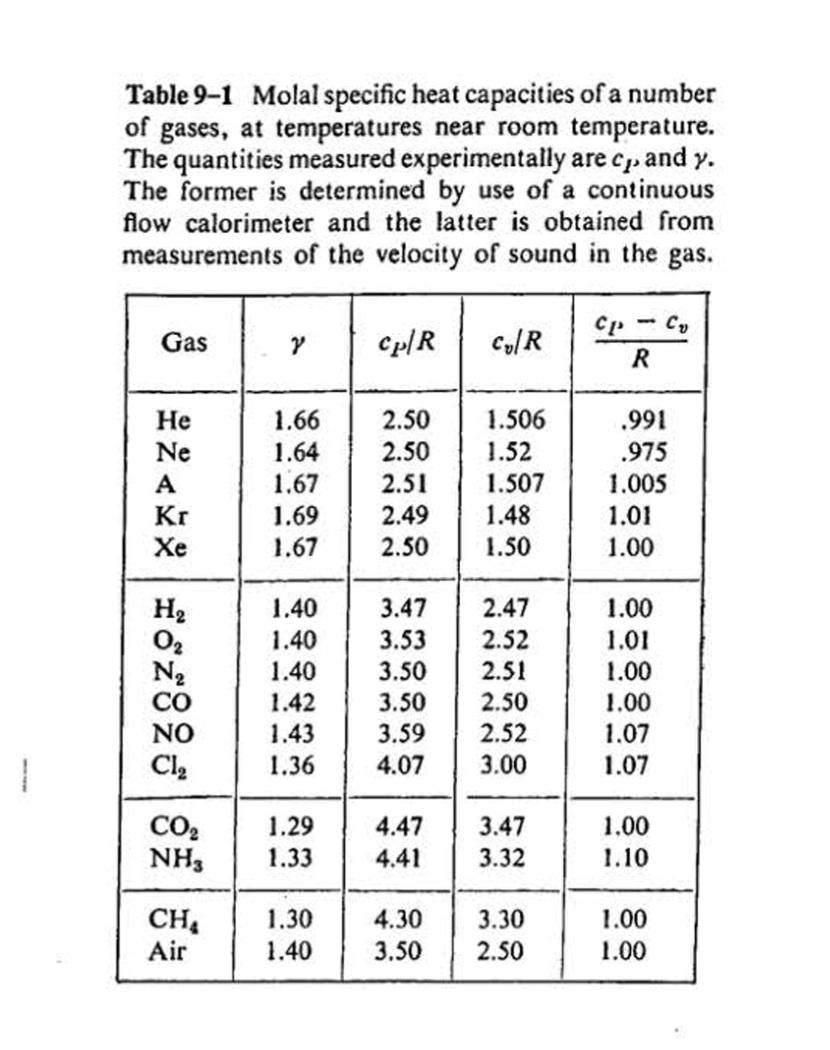
6-30 Carbon dioxide at an initial pressure of 100 atm and a temperature of 300 K under- goes an adiabatic free expansion in which the final volume is ten times the original volume. Find the change in temperature and the increase in specific entropy, assuming that CO, is (a) an ideal gas, (b) a van der Waals gas. (Use Tables 2-1 and 9-1 and make assumptions that seem reasonable.) any other Table 2-1 Constants a and b in van der Waals equation. Pin Nm,vin m kilomole-, Tin kelvins, R = 8.31 x 103 J kilomole- K-, a Substance (J m kilomole-) (m kilomole-) 3.44 x 10 0.0234 H2 24.8 .0266 138 .0318 CO2 366 .0429 H,0 580 .0319 Hg 292 .0055 Table 9-1 Molal specific heat capacities of a number of gases, at temperatures near room temperature. The quantities measured experimentally are cp. and y. The former is determined by use of a continuous flow calorimeter and the latter is obtained from measurements of the velocity of sound in the gas. Cp-C, Gas Cp/R Cy/R 1.66 2.50 1.506 .991 2.50 1.52 1.507 Ne 1.64 .975 A 1.67 2.51 1.005 Kr 1.69 2.49 1.48 1.01 1.67 2.50 1.50 1.00 H2 1.40 3.47 2.47 1.00 1.40 3.53 2.52 1.01 N2 CO 1.40 3.50 2.51 1.00 1.42 1.43 3.50 2.50 1.00 NO 3.59 2.52 1.07 Cl, 1.36 3.00 1.07 CO2 NH, 1.29 4.47 3.47 1.00 .33 4.41 3.32 1.10 CH Air 1.30 4.30 3.30 1.00 1.40 3.50 2.50 1.00
Step by Step Solution
★★★★★
3.40 Rating (162 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


