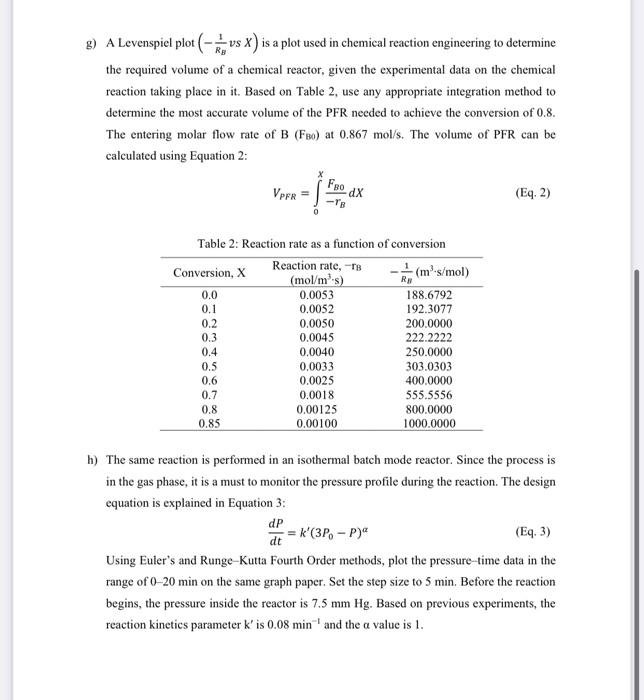
numeric method
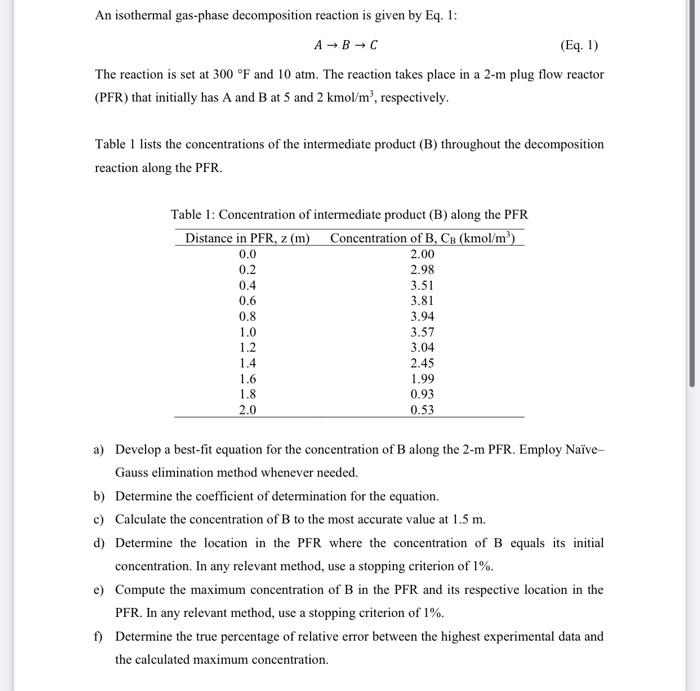
An isothermal gas-phase decomposition reaction is given by Eq. 1: ABC The reaction is set at 300F and 10atm. The reaction takes place in a 2m plug flow reactor (PFR) that initially has A and B at 5 and 2kmol/m3, respectively. Table 1 lists the concentrations of the intermediate product (B) throughout the decomposition reaction along the PFR. Table 1: Concentration of intermediate product (B) along the PFR a) Develop a best-fit equation for the concentration of B along the 2-m PFR. Employ NaveGauss elimination method whenever needed. b) Determine the coefficient of determination for the equation. c) Calculate the concentration of B to the most accurate value at 1.5m. d) Determine the location in the PFR where the concentration of B equals its initial concentration. In any relevant method, use a stopping criterion of 1%. e) Compute the maximum concentration of B in the PFR and its respective location in the PFR. In any relevant method, use a stopping criterion of 1%. f) Determine the true percentage of relative error between the highest experimental data and the calculated maximum concentration. g) A Levenspiel plot (RB1vsX) is a plot used in chemical reaction engineering to determine the required volume of a chemical reactor, given the experimental data on the chemical reaction taking place in it. Based on Table 2, use any appropriate integration method to determine the most accurate volume of the PFR needed to achieve the conversion of 0.8. The entering molar flow rate of B(FB) at 0.867mol/s. The volume of PFR can be calculated using Equation 2 : VPFR=0xrBFB0dX Table 2: Reaction rate as a function of conversion h) The same reaction is performed in an isothermal batch mode reactor. Since the process is in the gas phase, it is a must to monitor the pressure profile during the reaction. The design equation is explained in Equation 3: dtdP=k(3P0P)a Using Euler's and Runge-Kutta Fourth Order methods, plot the pressure-time data in the range of 0-20 min on the same graph paper. Set the step size to 5min. Before the reaction begins, the pressure inside the reactor is 7.5mmHg. Based on previous experiments, the reaction kinetics parameter k is 0.08min1 and the value is 1