Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Objectives: 1. Determine the empirical formula of a bydrated compound 2. Practice writing detailed observations in the laboratary 3. Practice calculating mass percent composition.
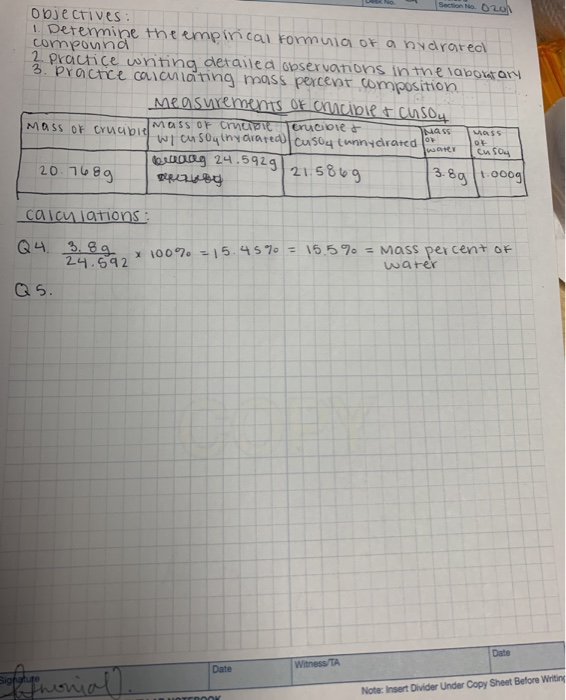



Objectives: 1. Determine the empirical formula of a bydrated compound 2. Practice writing detailed observations in the laboratary 3. Practice calculating mass percent composition. Mass of crucible. 20.7689 calculations: Q4. 3.89 24.592 Q5. Signature Sofymonial). badag 24.592g Measurements Of crucible & CuSO4 Mass of crucible crucible & w/ cu sog (ny arated) Cusoy (unnydrated. 21.5869 Section No. 020 Date NOTEBOOK x 100% = 15.45% = 15.5% = Mass percent of water Witness/TA Nass OF water Mass OF cu 504 3.8g 1.000g Date Note: Insert Divider Under Copy Sheet Before Writing Calculations: Determining Empirical Formulas Remember to include units on all numbers and recall significant figures! 1. Determine the initial mass of your hydrated copper (II) sulfate sample. 2. Determine the final mass of your anhydrous copper (II) sulfate that remained as your product. 3. Calculate the mass of water that was driven from your salt sample. 4. Calculate the mass percent of water in your hydrated copper (II) sulfate sample, and report it on the board to share your results with your classmates: Mass percent of water Mass water Mass hydrated salt -x 100% [Notice that this formula is different from the one given in the introduction. That is because the equation given in the introduction is for calculating the mass percent composition if you already know the chemical formula. The equation given here, on the other hand, is for calculating the mass percent composition from experimental data (since we do not yet know the chemical formula).] 5. Based on your answer to Q2, calculate the moles of anhydrous copper (II) sulfate that remained as your product. 6. Based on your answer to Q3, calculate the moles of water that were driven from your salt sample. 7. Using your answers from 5 and 6, determine the empirical formula for the hydrated salt and report it on the board to share your results with your classmates. Save spa Analysis & Discussion/Follow-up Questions: 1. Compare your results to those of the other groups. Are all the results the same? Should they be? Explain your reasoning. 2. Ask your instructor for the true empirical formula, and calculate the true mass percent of water from this (using the equation given in the introduction). 3. Compare the true mass percent of water to the results of the class. Discuss the class results in terms of their accuracy and precision. 4. Calculate the percent error for your results for the mass percent of water in the hydrated salt. Percent error True value - Experimental value] True value -x 100% 5. In Principles of Chemistry, any error less than 10% is acceptable. a. If your percent error is greater than 10%, write a few sentences describing the sources of error you would correct if the experiment were repeated. b. If your percent error is less than 10%, write a few sentences describing the good laboratory practices you used that contributed to your accurate results. Percent error True value Experimental value] -x 100% True value 5. In Principles of Chemistry, any error less than 10% is acceptable. a. If your percent error is greater than 10%, write a few sentences describing the sources of error you would correct if the experiment were repeated. b. If your percent error is less than 10%, write a few sentences describing the good laboratory practices you used that contributed to your accurate results. 6. Here, we determined the empirical formula of the hydrated salt sample. What is the difference between an empirical formula and a molecular formula? Conclusion: Write a brief conclusion addressing the main objectives of the lab and any major results. Be sure to relate how your results are relevant to the objectives!
Step by Step Solution
★★★★★
3.51 Rating (154 Votes )
There are 3 Steps involved in it
Step: 1
Lets go step by step to calculate the empirical formula of the hydrated copper II sulfate based on the given data Make sure to carry units through the calculations and use the correct number of signif...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


