Question
Octane (C8H18) at 25C is burned in the combustion chamber of a steam boiler with 20 percent excess air that also enters at 25C. The
Octane (C8H18) at 25C is burned in the combustion chamber of a steam boiler with 20 percent excess air that also enters at 25C. The products leave the combustion chamber at 500 K. Assuming combustion is complete, determine
(a) the percent exhaust losses and
(b) the boiler efficiency if other losses (such as incomplete com n and heat losses) amount to 2.5 percent.. Use Eq. (5-17) with the molar enthalpies of product gases from the ideal gas tables (Tables A-11 through A-16).
-This question need to be solved by balance the chemical equation then doing the Calculations of (q exhaust ) and boiler efficiency by the equations in the below photos
Note the chemical equation after balancing will be:
C8H18+15(O2+3.76N2)  8CO2+9H2O+2.5O2+56.4N2
8CO2+9H2O+2.5O2+56.4N2

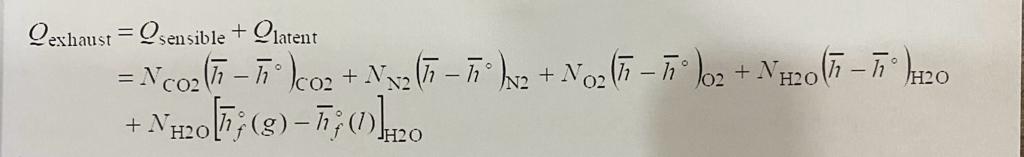 boiler=100 Exhaust losses - Other losses Qexhaust=Qsensible+Qlatent=NCO2(hh)CO2+NN2(hh)N2+NO2(hh)O2+NH2O(hh)H2O+NH2O[hf(g)hf(l)]H2O boiler=100 Exhaust losses - Other losses Qexhaust=Qsensible+Qlatent=NCO2(hh)CO2+NN2(hh)N2+NO2(hh)O2+NH2O(hh)H2O+NH2O[hf(g)hf(l)]H2O
boiler=100 Exhaust losses - Other losses Qexhaust=Qsensible+Qlatent=NCO2(hh)CO2+NN2(hh)N2+NO2(hh)O2+NH2O(hh)H2O+NH2O[hf(g)hf(l)]H2O boiler=100 Exhaust losses - Other losses Qexhaust=Qsensible+Qlatent=NCO2(hh)CO2+NN2(hh)N2+NO2(hh)O2+NH2O(hh)H2O+NH2O[hf(g)hf(l)]H2O Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


