Answered step by step
Verified Expert Solution
Question
1 Approved Answer
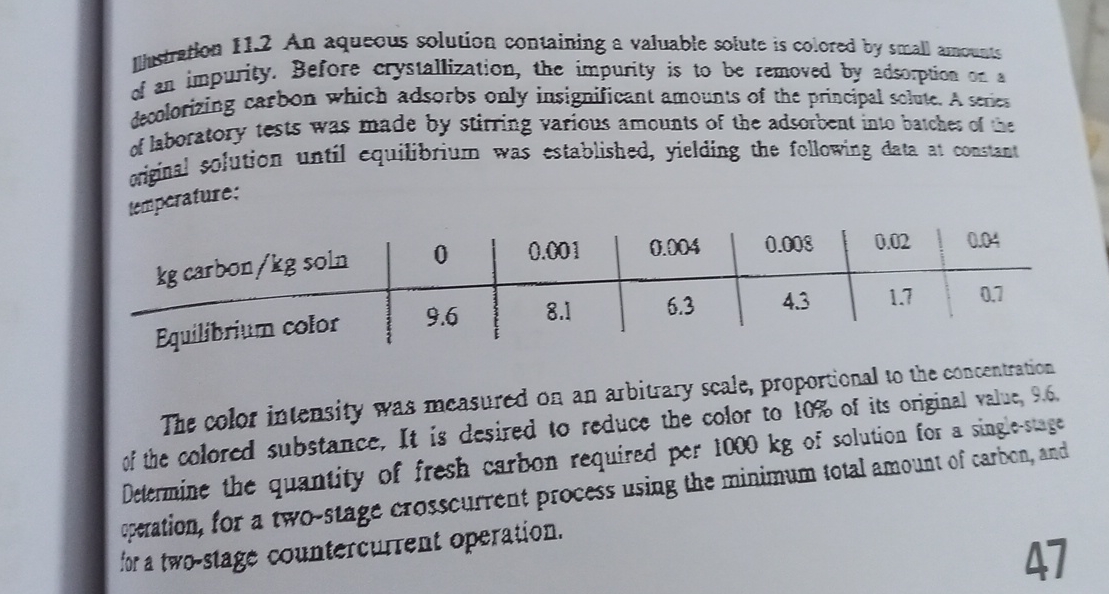
of an impurity. Before crystallization, the impurity is to be removed by adsoppion on a deoolorizing carbon which adsorbs only insignificant amounts of the principal
of an impurity. Before crystallization, the impurity is to be removed by adsoppion on a deoolorizing carbon which adsorbs only insignificant amounts of the principal solule. A serices of laboratory tests was made by stirring various amounts of the adsorbeat into batches of be original solution until equilibrium was established, yielding the following data at constant tertperature:
table carbon soln,Equilibrium color,
The color intensity was measured on an arbitrary scale, proportional to the concentration of the colored substance, It is desired to reduce the color to of its original value, Determine the quantity of fresh carbon required per of solution for a singestage opration, for a twostage crosscurtent process using the minimum total amount of carbon, and tor a twostage countercurtent operation.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


