(D) If the partial pressure of A in V2 is 20.132 kPa at time t, calculate the diffusion coefficient DAB in SI units? Two glass
 (D) If the partial pressure of A in V2 is 20.132 kPa at time t, calculate the diffusion coefficient DAB in SI units?
(D) If the partial pressure of A in V2 is 20.132 kPa at time t, calculate the diffusion coefficient DAB in SI units?
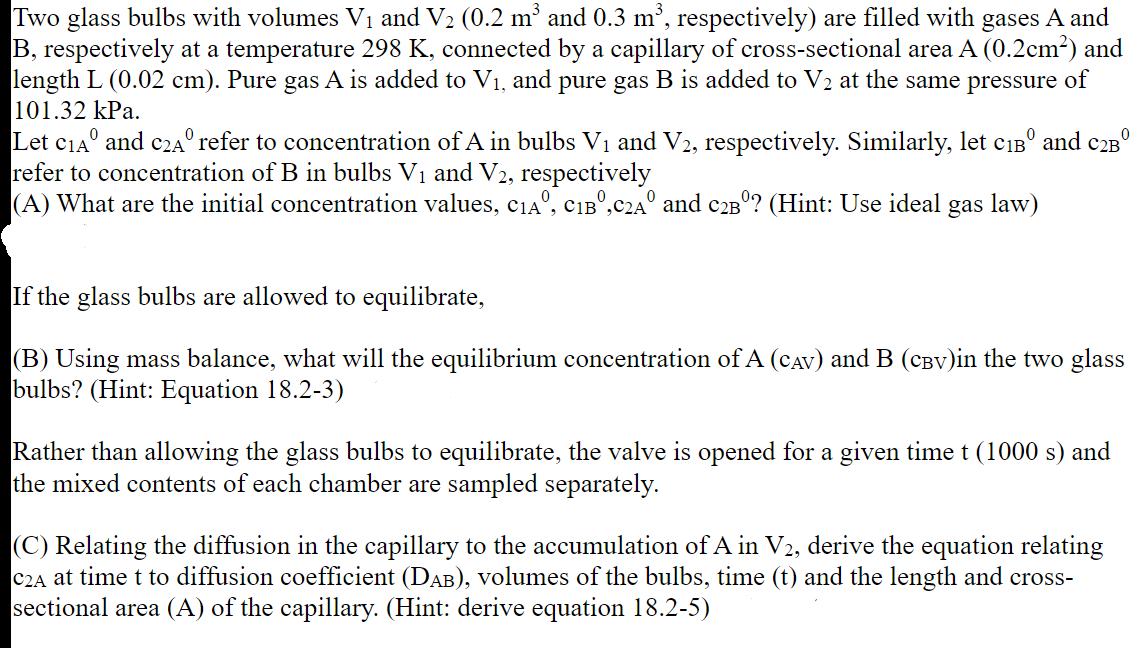
Two glass bulbs with volumes V and V (0.2 m and 0.3 m, respectively) are filled with gases A and B, respectively at a temperature 298 K, connected by a capillary of cross-sectional area A (0.2cm) and length L (0.02 cm). Pure gas A is added to V, and pure gas B is added to V2 at the same pressure of 101.32 kPa. Let CIA and C2A refer to concentration of A in bulbs V and V2, respectively. Similarly, let CB and C2B refer to concentration of B in bulbs V and V2, respectively 0 (A) What are the initial concentration values, CIA, CIB,C2A and C2B? (Hint: Use ideal gas law) If the glass bulbs are allowed to equilibrate, (B) Using mass balance, what will the equilibrium concentration of A (CAV) and B (CBV)in the two glass bulbs? (Hint: Equation 18.2-3) Rather than allowing the glass bulbs to equilibrate, the valve is opened for a given time t (1000 s) and the mixed contents of each chamber are sampled separately. (C) Relating the diffusion in the capillary to the accumulation of A in V2, derive the equation relating C2A at time t to diffusion coefficient (DAB), volumes of the bulbs, time (t) and the length and cross- sectional area (A) of the capillary. (Hint: derive equation 18.2-5)
Step by Step Solution
3.52 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


