Question
An environemtal aqeous samples has 515 g/mL of Gallium (III) ion and 323 g/mL of Mecury (II) ion, NaOH solution is slowly dropped into
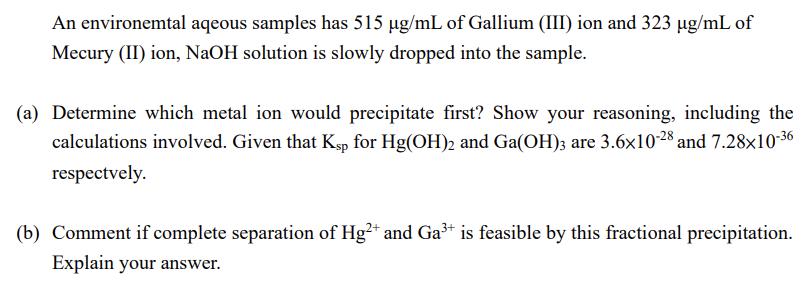
An environemtal aqeous samples has 515 g/mL of Gallium (III) ion and 323 g/mL of Mecury (II) ion, NaOH solution is slowly dropped into the sample. (a) Determine which metal ion would precipitate first? Show your reasoning, including the calculations involved. Given that Ksp for Hg(OH)2 and Ga(OH)3 are 3.6x10-28 and 7.28x10-36 respectively. (b) Comment if complete separation of Hg2+ and Ga+ is feasible by this fractional precipitation. Explain your answer.
Step by Step Solution
3.41 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Financial Statement Analysis
Authors: K. R. Subramanyam, John Wild
11th edition
78110963, 978-0078110962
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



