Question
On July 13, 1991, a train derailment resulted in a spill of approximately 13,000 gallons (1 US gallon = 3.785 litres) of a soil fumigant
On July 13, 1991, a train derailment resulted in a spill of approximately 13,000 gallons (1 US gallon = 3.785 litres) of a soil fumigant (sodium metam) to the Sacramento River, 70 km upstream from Shasta Lake. The spill killed almost all aquatic life in the river.
a) Assuming the spill acts acts as a single point source. How long does it take for the maximum contaminant concentration to get to Lake Shasta? (2 marks)
b) If Na+ acts as conservative (i.e., it is not transformed) tracer, calculate the maximum concentration (in g/L) at Lake Shasta. (2 marks) (Hint: remember that sodium only constitutes a fraction of the total mass of sodium metam, you will need to use molar masses)
c) Calculate the maximum concentration of metam that will be encountered at Lake Shasta if photolysis is the only mechanism of transformation. (2 marks)
d) Calculate the maximum concentration of metam that will be encountered Lake Shasta if photolysis and hydrolysis are both occurring. (2 marks)
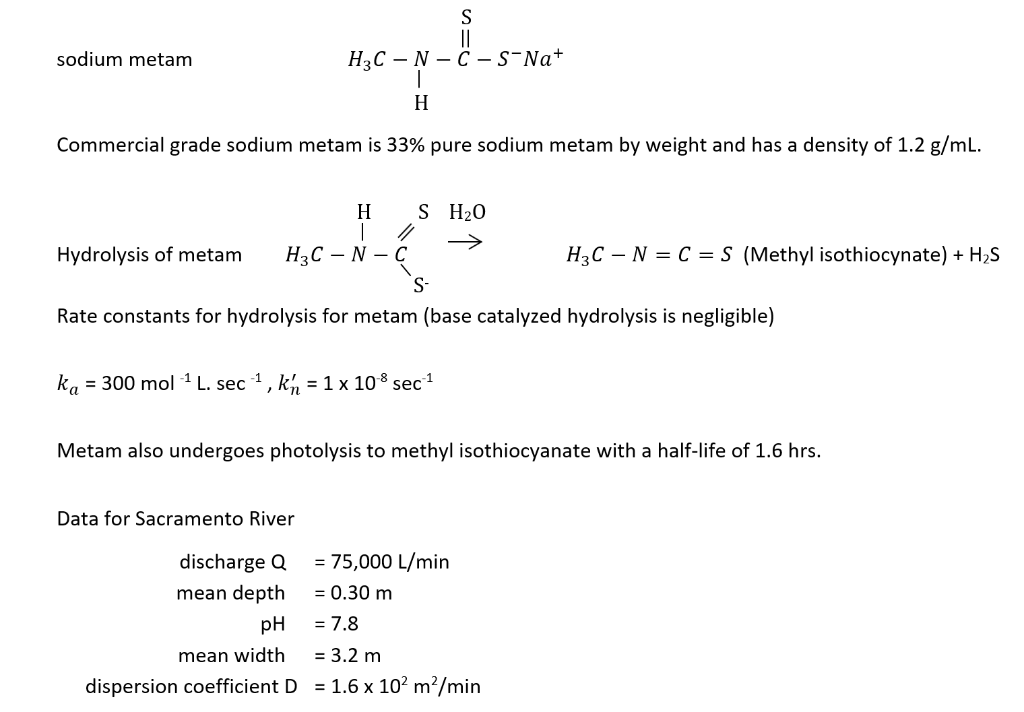
sodium metam Commercial grade sodium metam is 33% pure sodium metam by weight and has a density of 1.2g/mL. Hydrolysis of metam Rate constants for hydrolysis for metam (base catalyzed hydrolysis is negligible) ka=300mol1Lsec1,kn=1108sec1 Metam also undergoes photolysis to methyl isothiocyanate with a half-life of 1.6hrs. Data for Sacramento River dischargeQmeandepthpHmeanwidthdispersioncoefficientD=75,000L/min=0.30m=7.8=3.2m=1.6102m2/minStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


