Question
Jom is testing out a chemical process for the extraction of iron metal from ferric oxide. They performed the following 2-step reaction (excess Zn(s)


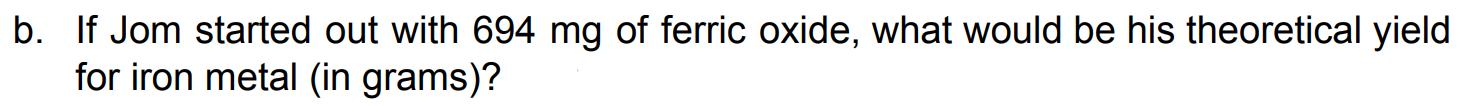

Jom is testing out a chemical process for the extraction of iron metal from ferric oxide. They performed the following 2-step reaction (excess Zn(s) is removed by adding acid): (MW: Fe=55.845 g/mol; O=15.999 g/mol) I. II. Nitric acid was added to ferric oxide to yield ferric nitrate Excess zinc dust was added to the ferric nitrate to yield metallic iron a. Write the balanced reactions from steps I and II, and identify the type of reaction for each step. If it is a redox reaction, identify the reducing and oxidizing agents. b. If Jom started out with 694 mg of ferric oxide, what would be his theoretical yield for iron metal (in grams)? c. Jom was able to extract 0.256 g of iron metal from his experiment in (b.). What is his percent yield?
Step by Step Solution
3.45 Rating (158 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Intermediate Accounting
Authors: James D. Stice, Earl K. Stice, Fred Skousen
17th Edition
032459237X, 978-0324592375
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



