Answered step by step
Verified Expert Solution
Question
1 Approved Answer
on peratura Study the following phase diagram of Substance X solid pressure (atro liquid gas 200 temperature (K) Use this diagram to answer the following
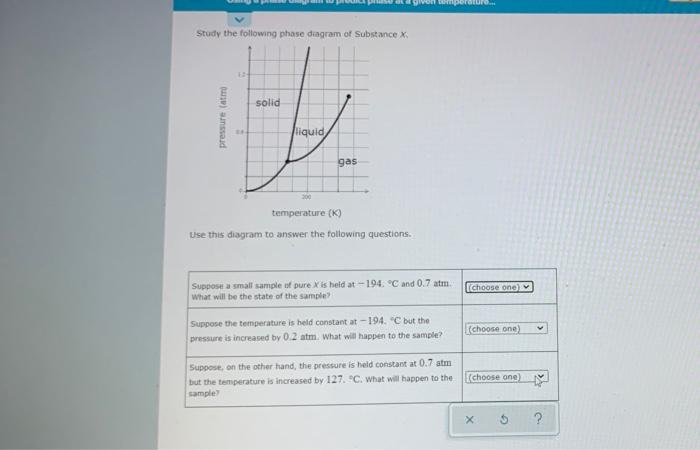 on peratura Study the following phase diagram of Substance X solid pressure (atro liquid gas 200 temperature (K) Use this diagram to answer the following questions Suppott a small sample of pure X is held at -194. Cand 0.7 atm What will be the state of the sample [choose one Suppose the temperature is held constant at -194. "C but the pressure is increased by 0.2 atm. What will happen to the sample? (choose one) Suppose, on the other hand, the pressure is held constant at 0.7 atm but the temperature is increased by 127. "C. What will happen to the sample) choose one) X 5
on peratura Study the following phase diagram of Substance X solid pressure (atro liquid gas 200 temperature (K) Use this diagram to answer the following questions Suppott a small sample of pure X is held at -194. Cand 0.7 atm What will be the state of the sample [choose one Suppose the temperature is held constant at -194. "C but the pressure is increased by 0.2 atm. What will happen to the sample? (choose one) Suppose, on the other hand, the pressure is held constant at 0.7 atm but the temperature is increased by 127. "C. What will happen to the sample) choose one) X 5

Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


