Answered step by step
Verified Expert Solution
Question
1 Approved Answer
One day during the pandemic, you decide to do a home experiment. You find some tetracycline in your medicine cabinet and you crush up
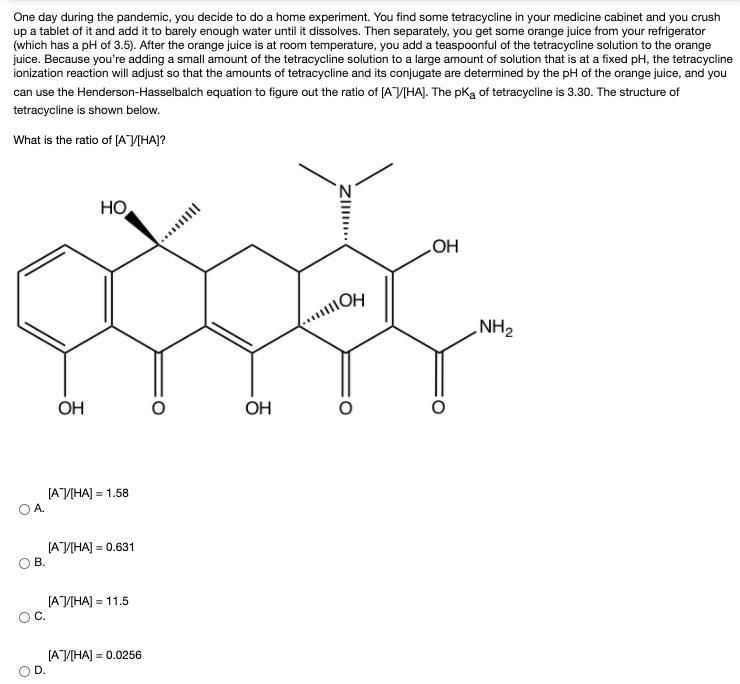
One day during the pandemic, you decide to do a home experiment. You find some tetracycline in your medicine cabinet and you crush up a tablet of it and add it to barely enough water until it dissolves. Then separately, you get some orange juice from your refrigerator (which has a pH of 3.5). After the orange juice is at room temperature, you add a teaspoonful of the tetracycline solution to the orange juice. Because you're adding a small amount of the tetracycline solution to a large amount of solution that is at a fixed pH, the tetracycline ionization reaction will adjust so that the amounts of tetracycline and its conjugate are determined by the pH of the orange juice, and you can use the Henderson-Hasselbalch equation to figure out the ratio of (AVIHAJ. The pKa of tetracycline is 3.30. The structure of tetracycline is shown below. What is the ratio of [AV[HA]? HO HO NH2 OH OH [AV[HA] = 1.58 [ ] - 0.631 . [ ] - 11.5 [AV[HA] = 0.0256 %3D ZI. II .
Step by Step Solution
★★★★★
3.39 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


