Question
One mole of a monatomic ideal gas undergoes four thermodynamic processes as shown schematically in the PV- diagram below. Among these four processes, one
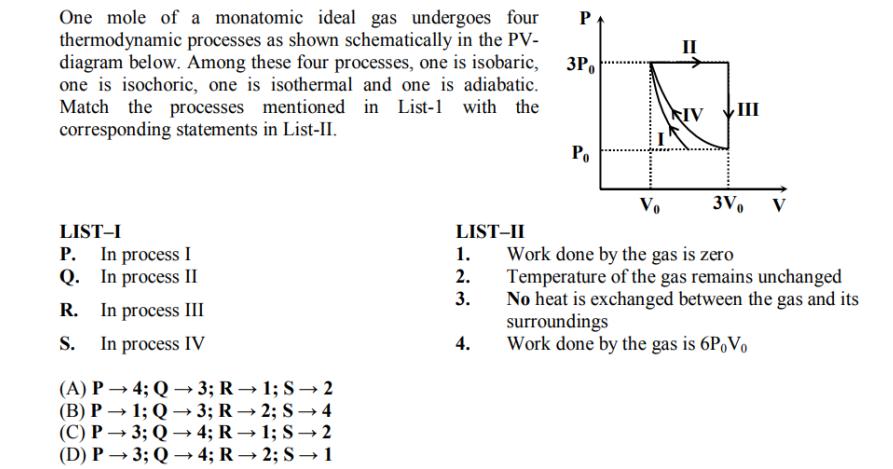
One mole of a monatomic ideal gas undergoes four thermodynamic processes as shown schematically in the PV- diagram below. Among these four processes, one is isobaric, one is isochoric, one is isothermal and one is adiabatic. Match the processes mentioned in List-1 with the corresponding statements in List-II. LIST-I P. In process I Q. In process II R. In process III S. In process IV (A) P4; Q3; R 1; S 2 (B) P 1; Q3; R2; S 4 (C) P3; Q 4; R-1; S2 (D) P3; Q4; R2; S 1 LIST-II 1. 2. 3. 4. P 3Po Po II Vo NIV 3Vo Work done by the gas is zero Temperature of the gas remains unchanged No heat is exchanged between the gas and its surroundings Work done by the gas is 6P Vo III V
Step by Step Solution
3.48 Rating (155 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Physics
Authors: John D. Cutnell, Kenneth W. Johnson
9th edition
470879564, 1118424840, 470879521, 9780470879566, 9781118424841, 978-0470879528
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



