Answered step by step
Verified Expert Solution
Question
1 Approved Answer
One mole of n-butane is contained in a cylinder-piston system. The system undergoes an isothermal expansion at constant pressure until equilibrium is reached. The
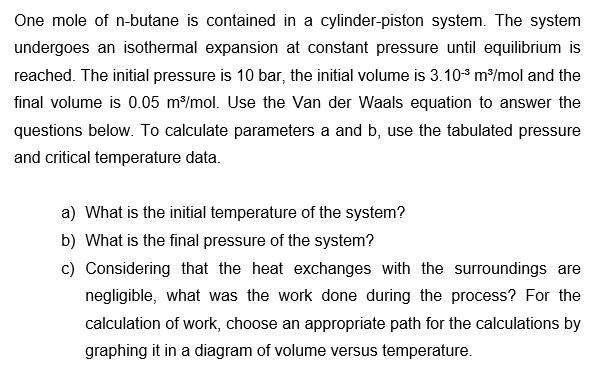
One mole of n-butane is contained in a cylinder-piston system. The system undergoes an isothermal expansion at constant pressure until equilibrium is reached. The initial pressure is 10 bar, the initial volume is 3.10- m/mol and the final volume is 0.05 m/mol. Use the Van der Waals equation to answer the questions below. To calculate parameters a and b, use the tabulated pressure and critical temperature data. a) What is the initial temperature of the system? b) What is the final pressure of the system? c) Considering that the heat exchanges with the surroundings are negligible, what was the work done during the process? For the calculation of work, choose an appropriate path for the calculations by graphing it in a diagram of volume versus temperature.
Step by Step Solution
★★★★★
3.43 Rating (159 Votes )
There are 3 Steps involved in it
Step: 1
a The vander waals gas equation is avnb nRT This can be rearranged to o...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


