Question
One of the chief concerns of using carbon-based fuels is the release of the greenhouse gas CO into the atmosphere. It would be best
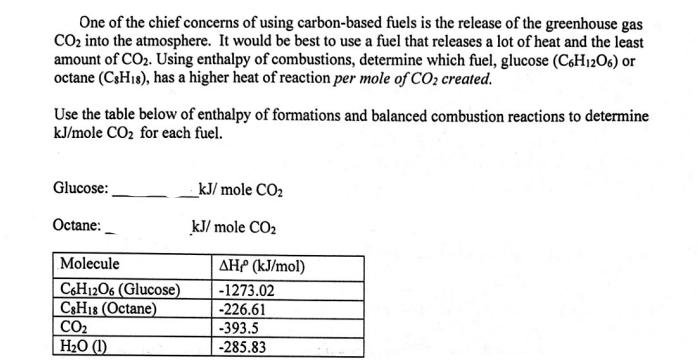
One of the chief concerns of using carbon-based fuels is the release of the greenhouse gas CO into the atmosphere. It would be best to use a fuel that releases a lot of heat and the least amount of CO. Using enthalpy of combustions, determine which fuel, glucose (C6H1206) or octane (CsH1s), has a higher heat of reaction per mole of CO2 created. Use the table below of enthalpy of formations and balanced combustion reactions to determine kJ/mole CO for each fuel. Glucose: Octane: Molecule C6H12O6 (Glucose) C8H18 (Octane) CO HO (1) kJ/mole CO kJ/mole CO AH (kJ/mol) -1273.02 -226.61 -393.5 -285.83
Step by Step Solution
3.44 Rating (157 Votes )
There are 3 Steps involved in it
Step: 1
The detailed ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
The Practice Of Statistics
Authors: Daren S. Starnes, Josh Tabor
6th Edition
978-1319113339
Students also viewed these Chemistry questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



