Question
One of the stages of making ammonia occurs in the inverter with the reaction: If initially 56 tons of nitrogen gas and 15 tons of
One of the stages of making ammonia occurs in the inverter with the reaction:
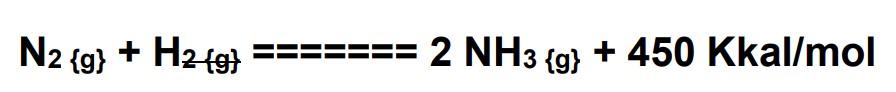
If initially 56 tons of nitrogen gas and 15 tons of hydrogen gas were reacted in a cylinder with a volume of 1000 liters, when 50% of the nitrogen gas reacted the system experienced an equilibrium. a. Determine the composition of the gases in the balanced system b. Determine the value of the equilibrium constant Kc c. If at that time the condition of 1 mole of gas was under pressure of 25 atm, determine the value of the constant Kp d. How does it affect the production of ammonia if the ammonia that is formed is released into other tubes? e. What happens if the temperature is raised from the initial level?
N2{g}+H2{g}======2NH3{g}+450Kkal/molStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


