Answered step by step
Verified Expert Solution
Question
1 Approved Answer
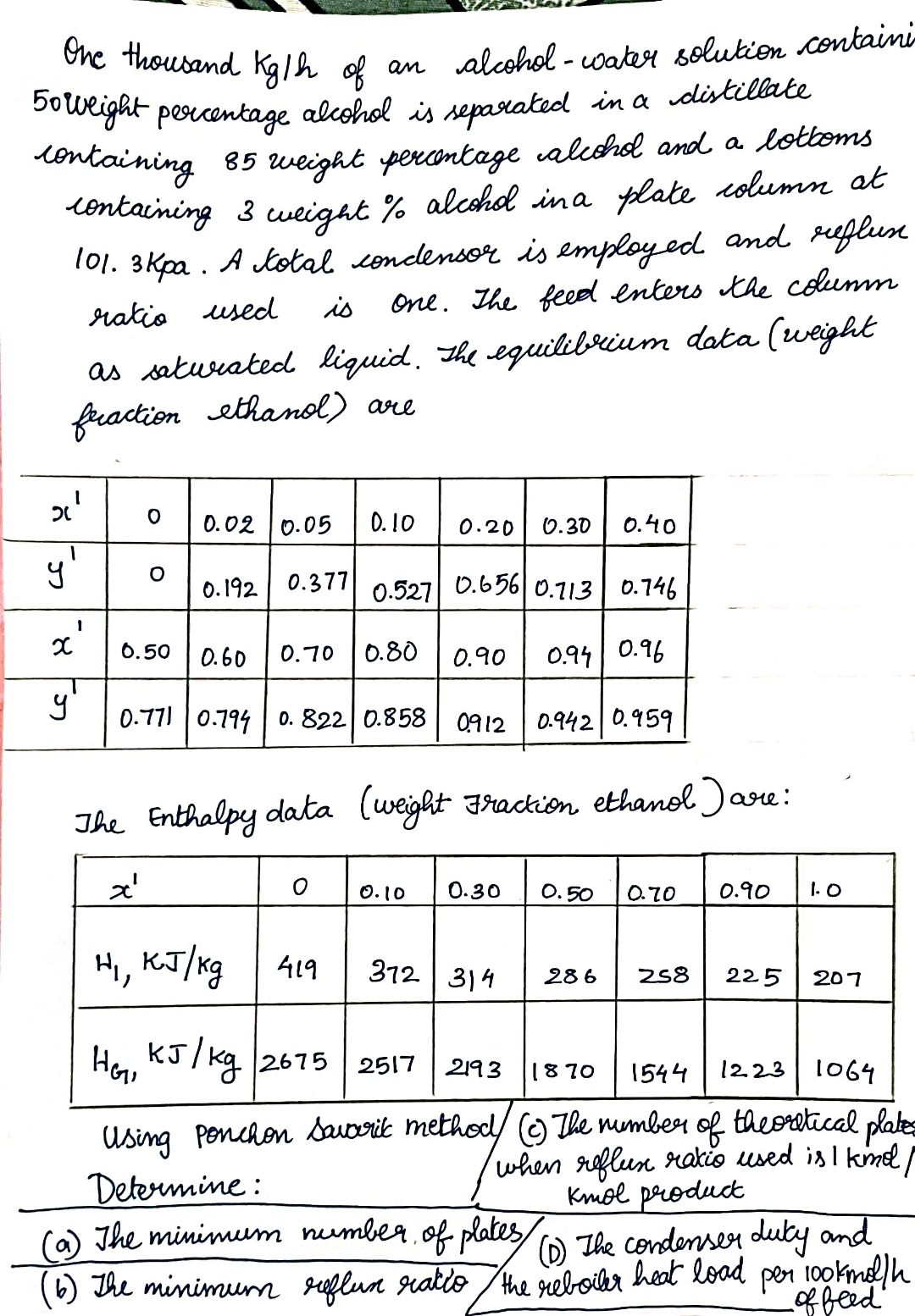
One thousand K g h of an alcohol - water solution containi 5 0 weight percentage alcohol is separated in a distillate containing 8 5
One thousand of an alcoholwater solution containi weight percentage alcohol is separated in a distillate containing weight percentage alcohol and a lotboms containing weight alcohol in a plate column at Kpa. A total condensor is employed and reflux ratio used is one. The feed enters the columm as satwrated liquid. The equilibrium data weight fraction ethanol are
table
The Enthalpy data weight Fraction ethanol are:
table
Using ponchon savaric methodc The number of theoretical plate
Determine: when reflux rakio used is ol kmol product
a The minimum number of platesD The condenser duty and
b The minimum reflun ratio the reboibr heat load per kmo of beed
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


