Answered step by step
Verified Expert Solution
Question
1 Approved Answer
One way to produce formaldehyde (HCHO) is via the partial oxidation of methanol (CH3OH). However, when methanol reacts with oxygen, some of the methanol
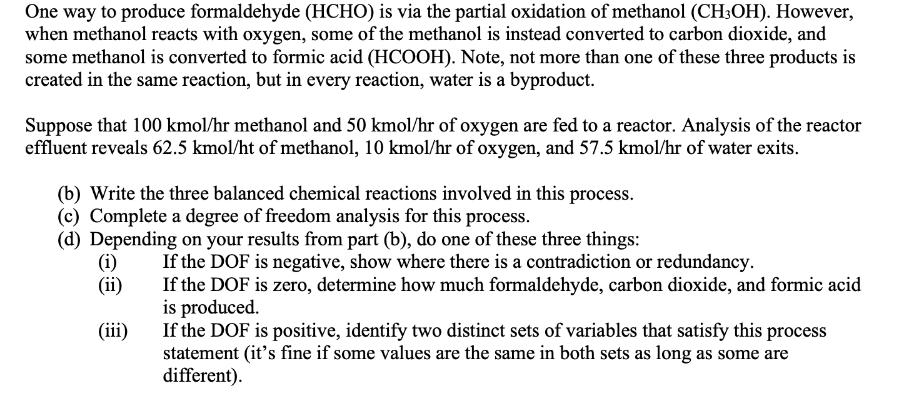
One way to produce formaldehyde (HCHO) is via the partial oxidation of methanol (CH3OH). However, when methanol reacts with oxygen, some of the methanol is instead converted to carbon dioxide, and some methanol is converted to formic acid (HCOOH). Note, not more than one of these three products is created in the same reaction, but in every reaction, water is a byproduct. Suppose that 100 kmol/hr methanol and 50 kmol/hr of oxygen are fed to a reactor. Analysis of the reactor effluent reveals 62.5 kmol/ht of methanol, 10 kmol/hr of oxygen, and 57.5 kmol/hr of water exits. (b) Write the three balanced chemical reactions involved in this process. (c) Complete a degree of freedom analysis for this process. (d) Depending on your results from part (b), do one of these three things: (i) (ii) (iii) If the DOF is negative, show where there is a contradiction or redundancy. If the DOF is zero, determine how much formaldehyde, carbon dioxide, and formic acid is produced. If the DOF is positive, identify two distinct sets of variables that satisfy this process statement (it's fine if some values are the same in both sets as long as some are different).
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


