Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Only correct letter I'll rate your answers One mole of an ideal gas at a temperature of 298 K and pressure of 3.0 bar expands



Only correct letter I'll rate your answers
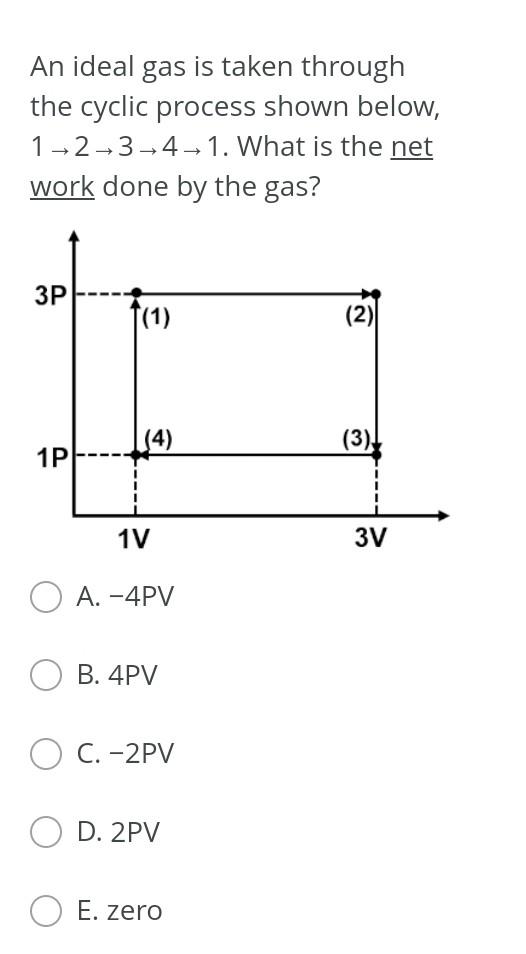
One mole of an ideal gas at a temperature of 298 K and pressure of 3.0 bar expands isothermally against a constant external pressure of 1.0 bar until the final pressure is 1.0 bar. The work done by the gas, w, is: O A. -1.7 kJ O B.-3.5 kJ OC. -0.8 kg O D.-4.4 kJ O E. -2.6 kJ A sample of 3.00 moles of an ideal gas, with CV,m = 12.47] mol-? K-1, at an initial temperature and pressure of 298 K and 1.00 bar, is adiabatically and reversibly compressed until the final pressure is 2.00 bar. The final temperature of the gas is: O A. 393 K B. 382 K C. 416 K O D. 404 K E. 428 K Consider the combustion of one mole of liquid cyclopentane (C5H10): C5H10 (1) + (15/2) O2 (g) + 5 CO2 (g) + 5 H20 (g) for which Ar H = -3071.4 kJ mol-1 at 298 K. The value of ArU at 298 Kis: A. -3077.6 kJ mol-1 B.-3072.4 kJ mol-1 C.-3068.2 kJ mol-1 O D.-3070.2 kJ mol-1 E. -3074.4 kJ mol-1 An ideal gas is taken through the cyclic process shown below, 1-2-3-4-1. What is the net work done by the gas? 3P |(1) (2) (4) (3) 1P 1V 3V O A. -4PV B. 4PV O C. -2PV O D. 2PV O E. zeroStep by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


