Answered step by step
Verified Expert Solution
Question
1 Approved Answer
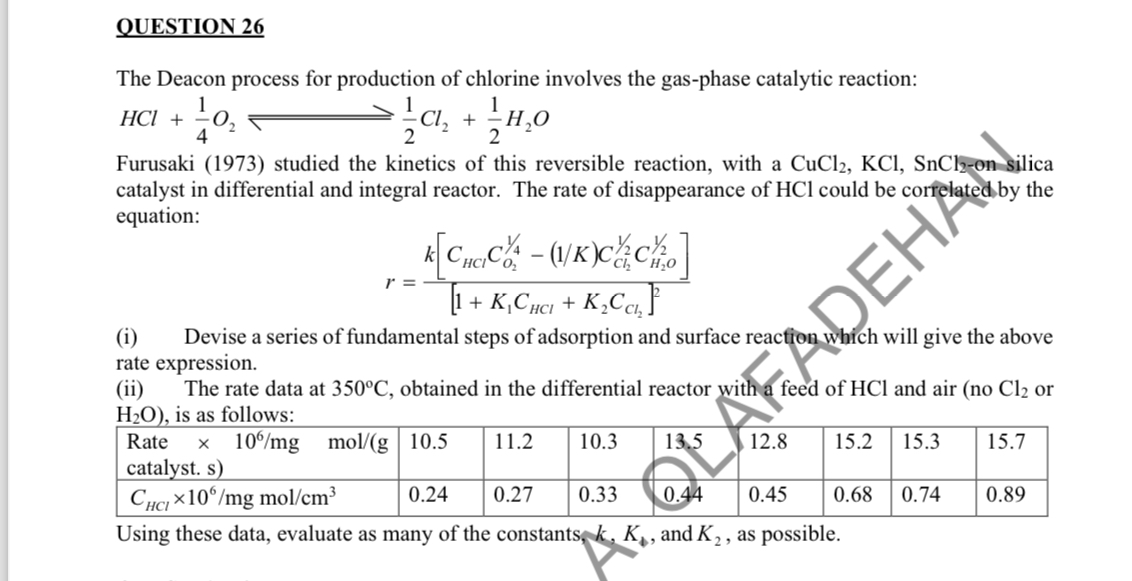
OUESTION 2 6 The Deacon process for production of chlorine involves the gas - phase catalytic reaction: H C l + 1 4 O 2
OUESTION
The Deacon process for production of chlorine involves the gasphase catalytic reaction:
Furusaki studied the kinetics of this reversible reaction, with a on silica catalyst in differential and integral reactor. The rate of disappearance of could be correlated by the equation:
i Devise a series of fundamental steps of adsorption and surface reaction which will give the above rate expression.
ii The rate data at obtained in the differential reactor with feed of and air no or is as follows:
tabletableRate
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


