Answered step by step
Verified Expert Solution
Question
1 Approved Answer
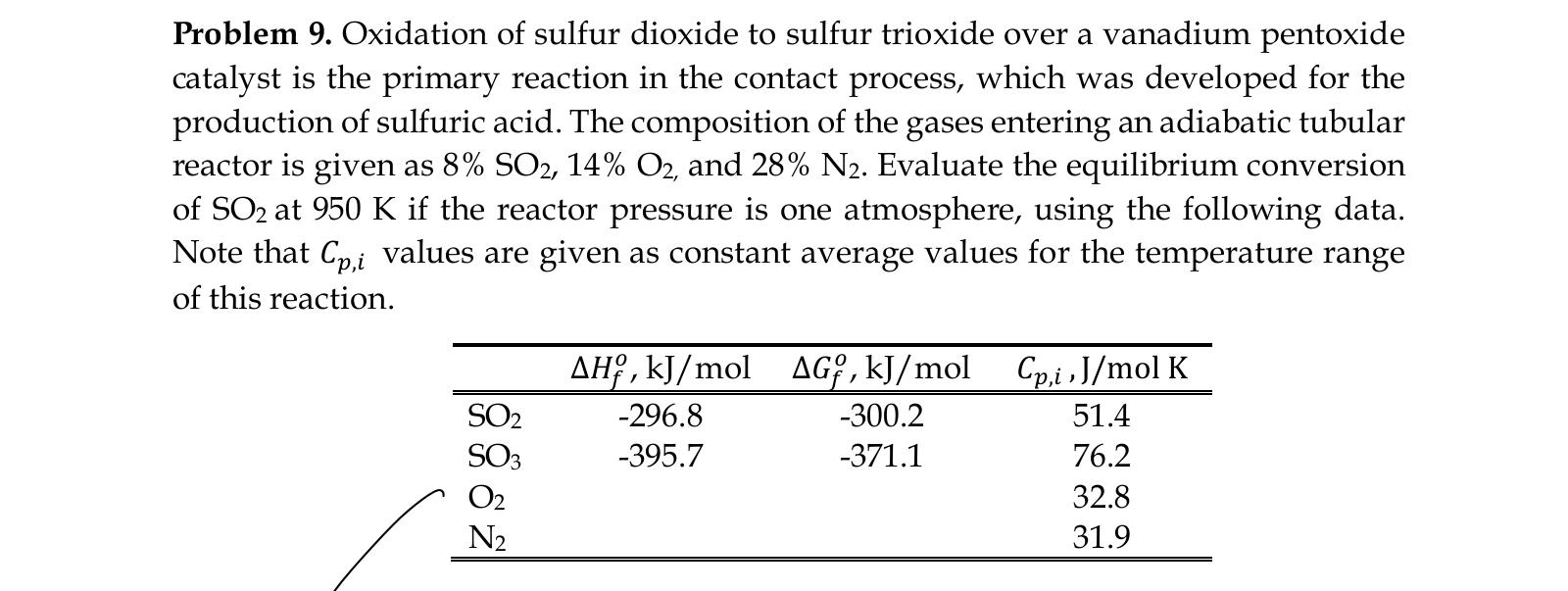
Oxidation of sulfur dioxide to sulfur trioxide over a vanadium pentoxide catalyst is the primary reaction in the contact process, which was developed for the
Oxidation of sulfur dioxide to sulfur trioxide over a vanadium pentoxide
catalyst is the primary reaction in the contact process, which was developed for the
production of sulfuric acid. The composition of the gases entering an adiabatic tubular
reactor is given as SO O and N Evaluate the equilibrium conversion
of SO at K if the reactor pressure is one atmosphere, using the following data.
Note that values are given as constant average values for the temperature range
of this reaction.
Problem Oxidation of sulfur dioxide to sulfur trioxide over a vanadium pentoxide catalyst is the primary reaction in the contact process, which was developed for the production of sulfuric acid. The composition of the gases entering an adiabatic tubular reactor is given as and Evaluate the equilibrium conversion of at if the reactor pressure is one atmosphere, using the following data. Note that values are given as constant average values for the temperature range of this reaction.
tableolK
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


