Answered step by step
Verified Expert Solution
Question
1 Approved Answer
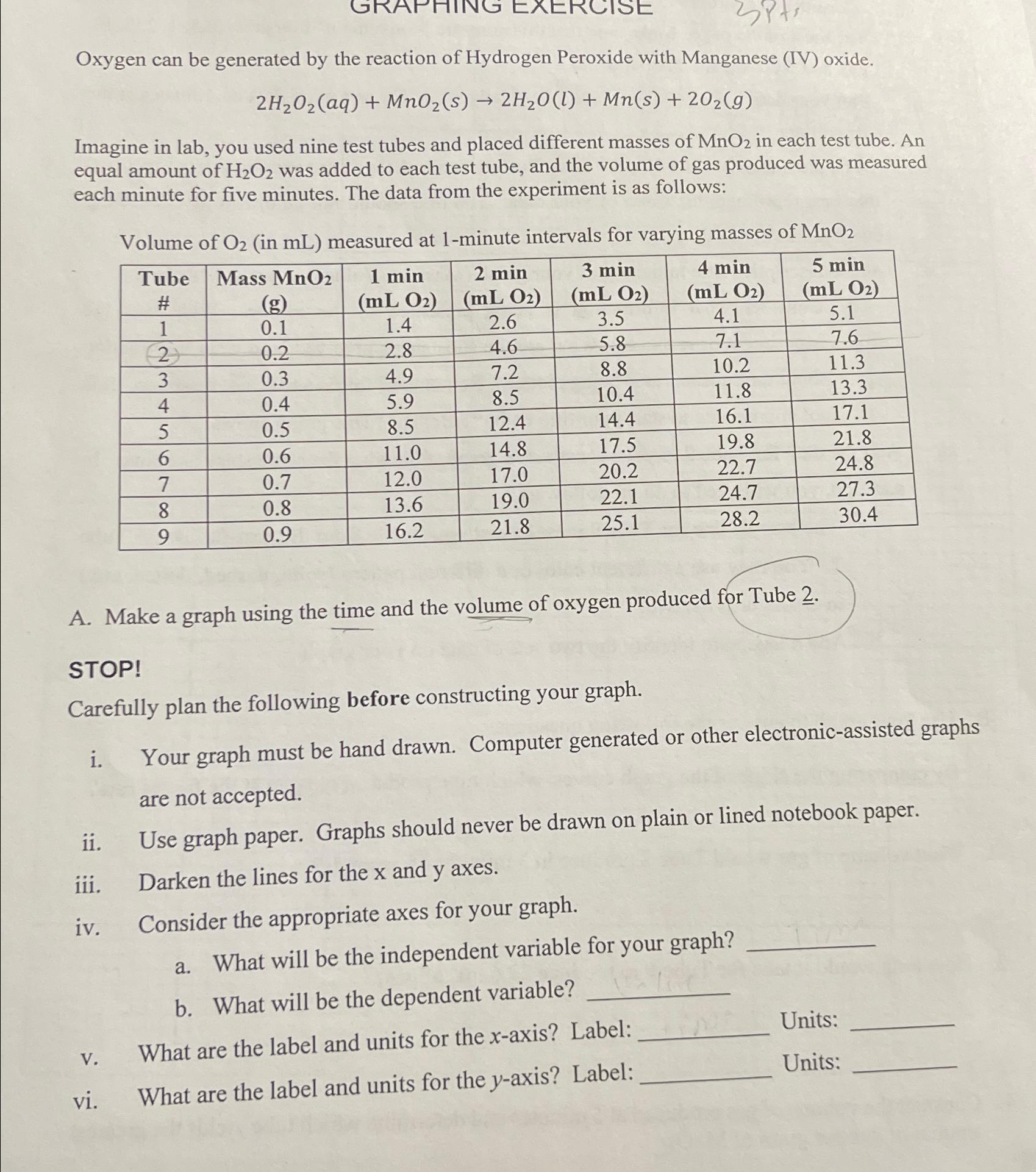
Oxygen can be generated by the reaction of Hydrogen Peroxide with Manganese ( IV ) oxide. 2 H 2 O 2 ( a q )
Oxygen can be generated by the reaction of Hydrogen Peroxide with Manganese IV oxide.
Imagine in lab, you used nine test tubes and placed different masses of in each test tube. An equal amount of was added to each test tube, and the volume of gas produced was measured each minute for five minutes. The data from the experiment is as follows:
Volume of in measured at minute intervals for varying masses of
tabletableTube#
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


