Answered step by step
Verified Expert Solution
Question
1 Approved Answer
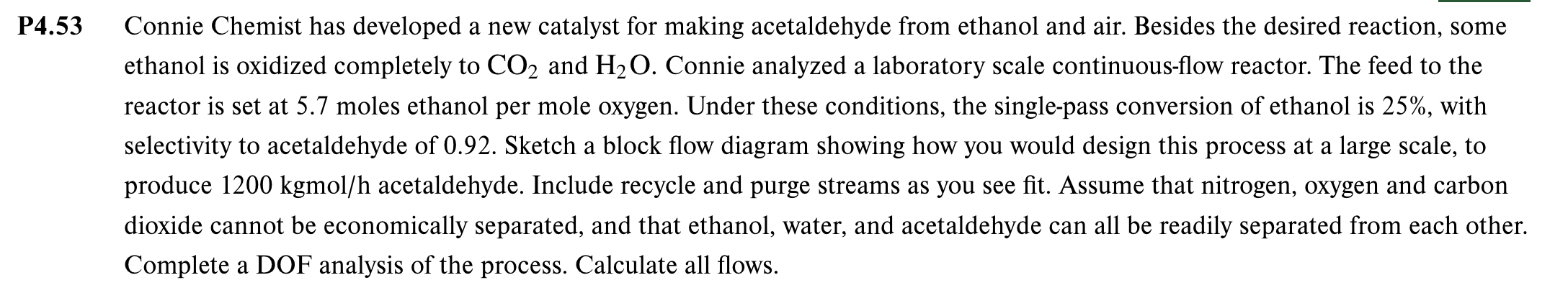
P 4 . 5 3 Connie Chemist has developed a new catalyst for making acetaldehyde from ethanol and air. Besides the desired reaction, some ethanol
P Connie Chemist has developed a new catalyst for making acetaldehyde from ethanol and air. Besides the desired reaction, some
ethanol is oxidized completely to and Connie analyzed a laboratory scale continuousflow reactor. The feed to the
reactor is set at moles ethanol per mole oxygen. Under these conditions, the singlepass conversion of ethanol is with
selectivity to acetaldehyde of Sketch a block flow diagram showing how you would design this process at a large scale, to
produce kgmo acetaldehyde. Include recycle and purge streams as you see fit. Assume that nitrogen, oxygen and carbon
dioxide cannot be economically separated, and that ethanol, water, and acetaldehyde can all be readily separated from each other.
Complete a DOF analysis of the process. Calculate all flows.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


