Question
P PA Lvap k Relative humidity = 17 - 21/) = 50% T mass of air molecules m = 28.8 x 10- kg/mole P
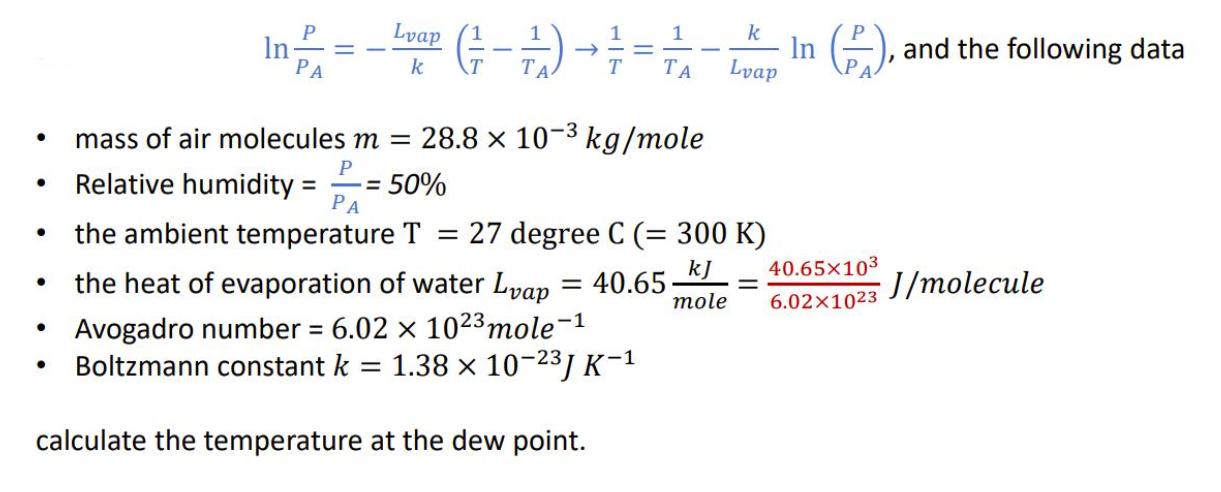
P PA Lvap k Relative humidity = 17 - 21/) = 50% T mass of air molecules m = 28.8 x 10- kg/mole P PA 1 the heat of evaporation of water Lvap = 40.65 Avogadro number = 6.02 x 1023 mole-1 Boltzmann constant k = 1.38 10-2J K- calculate the temperature at the dew point. k Lvap the ambient temperature T = 27 degree C (= 300 K) In (2), and the following data kJ 40.65103 mole 6.021023 = J/molecule
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
University Physics with Modern Physics
Authors: Hugh D. Young, Roger A. Freedman, A. Lewis Ford
13th edition
321696867, 978-0321696861
Students also viewed these Physics questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



