Answered step by step
Verified Expert Solution
Question
1 Approved Answer
P02. (30 pts) A two phase liquid-vapor mixture of HO, initially at equilibrium in state 1 with p = 1.5bar (150 kPa) with a

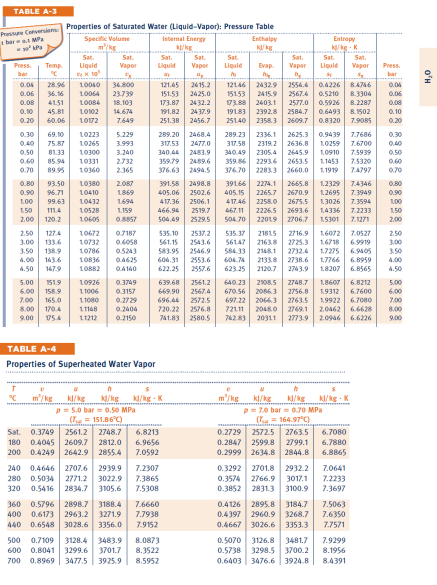
P02. (30 pts) A two phase liquid-vapor mixture of HO, initially at equilibrium in state 1 with p = 1.5bar (150 kPa) with a quality of x = 75% is contained in a sealed tank with rigid walls. The mass of HO is m = 5 kg. a) (5) Determine the volume of the tank, V, in m. b) (5) Determine the total internal energy of the liquid-vapor mixture at state 1 Next, heat is added to the tank until the phase of the water in the tank becomes state 2, the saturated vapor state. c) (5) determine the pressure of the state 2 d) (5) Determine the temperature of the water at state 2, Tz in degrees K e) (5) determine how much heat must be added to the tank to obtain state 2 f) (5) the heating continues untill the water in the tank reaches state 3 with a pressure p3-5bar. What is the temperature of the water in the tank at state 3, T3? TABLE A-3 Pressure Conversions Properties of Saturated Water (Liquid-Vapor): Pressure Table bar MP Specific Volume Internal Energy Enthalpy Entropy k/kg K Sat. Sat. Sat. Sat Sat Press Temp. Liquid Vapor Liquid Vapor bar r x10 5x 0.04 28.96 10040 34.800 0.06 36.16 10064 0.08 41.51 10084 0.10 45.81 23.739 18.103 10102 14.674 121.45 2415.2 151.53 2425.0 173.87 2432.2 191.82 2437.9 0.20 60.06 10172 7.649 0.30 69.10 10223 5.229 0.40 75.87 10265 3.993 0.50 81.33 10300 3.240 0.60 85.94 10331 2732 0.70 89.95 10360 2.365 251.38 2456.7 289.20 2468.4 317.53 2477.0 340.44 2483.9 359.79 2489.6 376.63 2494.5 Liquid Evap h hie 12146 2432.9 2554.4 0.4226 15153 2415.9 2567.4 0.5210 173.88 2403.1 2577.0 0.5926 19183 2392.8 2584.7 0.6493 8.1502 25140 2358.3 2609.7 289.23 2336.1 2625.3 0.9439 Sat. Vapor Sat Liquid Vapor Sat. Press bar 8.4746 0.04 8.3304 8.2287 0.06 0.08 0.10 0.8320 7.9085 0.20 7.7686 0.30 317.58 2319.2 2636.8 1.0259 340.49 2305.4 2645.9 1.0910 7.5939 0.50 359.86 2293.6 2653.5 1.1453 7.5320 0.60 376.70 2283.3 2660.0 1.1919 7.4797 0.70 7.6700 0.40 0.80 93.50 10380 2.087 391.58 0.90 96.71 10410 1869 405.06 2498.8 2502.6 1.00 1.50 99.63 10432 1.694 417.36 2506.1 111.4 10528 1.159 2.00 120.2 10605 0.8857 466.94 2519.7 504.49 2529.5 39166 2274.1 2665.8 1.2329 7.4346 405.15 2265.7 2670.9 1.2695 7.3949 417.46 2258.0 2675.5 1.3026 7.3594 467.11 22265 2693.6 1.4336 7.2233 504.70 22019 2706.7 1.5301 7.1271 0.80 0.90 1,00 1.50 2.00 2.50 127.4 10672 0.7187 3.00 133.6 10732 0.6058 3.50 138.9 10786 0.5243 535.10 2537.2 535.37 21815 561.15 2543.6 56147 2163.8 583.95 2546.9 584.33 2148.1 2716.9 2725.3 1.6072 7.0527 2.50 1.6718 6.9919 3.00 2732.4 1.7275 6.9405 3.50 4.00 143.6 10836 0.4625 604.31 4.50 147.9 10882 0.4140 2553.6 604.76 2133.8 622.25 2557.6 623.25 2120.7 2738.6 1.7766 6.8959 4.00 2743.9 1.8207 6.8565 4.50 5.00 151.9 10926 0.3749 639.68 2561.2 6.00 158.9 11006 0.3157 669.90 2567.4 7.00 165.0 11080 0.2729 8.00 170.4 11148 0.2404 9.00 175.4 11212 0.2150 696.44 2572.5 720.22 2576.8 741.83 2580.5 640.23 2108.5 1.8607 670.56 2086.3 2756.8 1.9312 697.22 2066.3 2763.5 1.9922 721.11 2048.0 2769.1 742.83 20311 2773.9 2748.7 6.8212 5.00 6.7600 6.00 6.7080 7.00 2.0462 6.6628 8.00 2.0946 6.6226 9.00 TABLE A-4 Properties of Superheated Water Vapor m/kg h kJ/kg kJ/kg-K m/kg p=5.0 bar=0.50 MPa (T=151.86C) kJ/kg kl/kg p=7.0 bar = 0.70 MPa (T=164.97C) kl/kg - K Sat. 0.3749 2561.2 2748.7 6.8213 180 0.4045 2609.7 2812.0 6.9656 200 0.4249 2642.9 2855.4 7.0592 0.2729 2572.5 2763.5 6.7080 320 240 0.4646 2707.6 2939.9 280 0.5034 2771.2 3022.9 0.5416 2834.7 3105.6 7.2307 7.3865 7.5308 0.2847 2599.8 2799.1 0.2999 2634.8 2844.8 0.3292 2701.8 2932.2 7.0641 0.3574 2766.9 3017.1 7.2233 0.3852 2831.3 3100.9 7.3697 6.7880 6.8865 360 0.5796 2898.7 3188.4 7.6660 440 400 0.6173 2963.2 3271.9 7.7938 0.6548 3028.6 3356.0 7.9152 0.4126 2895.8 3184.7 7.5063 0.4397 2960.9 3268.7 7.6350 0.4667 3026.6 3353.3 7.7571 500 0.7109 3128.4 3483.9 8.0873 600 0.8041 3299.6 3701.7 8.3522 700 0.8969 3477.5 3925.9 8.5952 0.5070 3126.8 3481.7 7.9299 0.5738 3298.5 3700.2 8.1956 0.6403 3476.6 3924.8 8.4391 HO
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


