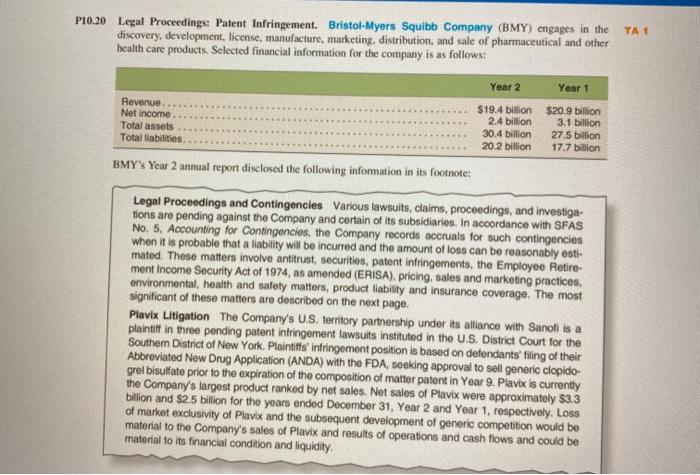
P10.20 Legal Proceedings: Patent Infringement. Bristol-Myers Squibb Company (BMY) engages in the discovery, development, license, manufacture, marketing, distribution, and sale of pharmaceutical and other health care products. Selected financial information for the company is as follows: TA 1 Year 2 Year 1 Revenue Net Income Total assets Total liabilities $19.4 billion 2.4 billion 30.4 billion 20.2 billion $20.9 billion 3.1 billion 27.5 billion 17.7 billion BMY's Year 2 annual report disclosed the following information in its footnote: Legal Proceedings and Contingencies Various lawsuits, claims, proceedings, and investiga- tions are pending against the Company and certain of its subsidiaries. In accordance with SFAS No. 5, Accounting for Contingencies, the Company records accruals for such contingencies when it is probable that a liability will be incurred and the amount of loss can be reasonably esti- mated. These matters involve antitrust, securities, patent infringements, the Employee Retire- ment Income Security Act of 1974, as amended (ERISA), pricing, sales and marketing practices. environmental health and safety matters, product liability and insurance coverage. The most significant of these matters are described on the next page. Plavix Litigation The Company's U.S. territory partnership under its alliance with Sanofi is a plaintiff in three pending patent infringement lawsuits instituted in the U.S. District Court for the Southern District of New York. Plaintiffs infringement position is based on defendants filing of their Abbreviated New Drug Application (ANDA) with the FDA, seeking approval to sell generic clopido- grel bisulfate prior to the expiration of the composition of matter patent in Year 9. Plavix is currently the Company's largest product ranked by net sales. Net sales of Plavix were approximately $3.3 billion and $2.5 billion for the years ended December 31, Year 2 and Year 1, respectively. Loss of market exclusivity of Plavix and the subsequent development of generic competition would be material to the Company's sales of Plavix and results of operations and cash flows and could be material to its financial condition and liquidity Legal Proceedings and Contingencies Various lawsuits, claims, proceedings, and investiga- tions are pending against the Company and certain of its subsidiaries. In accordance with SFAS No. 5, Accounting for Contingencies, the Company records accruals for such contingencies when it is probable that a liability will be incurred and the amount of loss can be reasonably esti- mated. These matters involve antitrust, securities, patent infringements, the Employee Retire- ment Income Security Act of 1974, as amended (ERISA), pricing, sales and marketing practices, environmental health and safety matters, product liability and insurance coverage. The most significant of these matters are described on the next page. Plavix Litigation The Company's U.S. territory partnership under its alliance with Sanofi is a plaintiff in three pending patent infringement lawsuits instituted in the U.S. District Court for the Southern District of New York. Plaintiffs infringement position is based on defendants' filing of their Abbreviated New Drug Application (ANDA) with the FDA, seeking approval to sell generic clopido grel bisultate prior to the expiration of the composition of matter patent in Year 9. Plavix is currently the Company's largest product ranked by net salos. Net sales of Plavix were approximately $3.3 billion and $2.5 billion for the years ended December 31, Year 2 and Year 1, respectively. LOSS of market exclusivity of Plavix and the subsequent development of generic competition would be material to the Company's sales of Plavix and results of operations and cash flows and could be material to its financial condition and liquidity, Required 1. Historically, the loss of patent protection on a brand name pharmaceutical product has resulted in a 70 percent decline in product sales. Assuming that (A) BMY patent protection on Plavix is lost as of the beginning of Year 3. (b) revenue and earnings for Year 3 would, in the absence of the loss of patent protection, have equaled those of Year 2, and (c) the after-tax margin on Plavix is 60 percent, estimate BMY'S revenue and comings for Year 3. How material are these financial effects? 2 BMY'S Plavix patent is carried on its balance as an intangible asset valued at $120 million. In the event of patent protection loss, how should the company reflect this in its financial statements? 3. How should the capital market react to (a) the disclosure that a patent infringement suit involving Plavix had been filed against BMY and (b) the disclosure that the suit had been lost (won)? D1031 - P10.20 Legal Proceedings: Patent Infringement. Bristol-Myers Squibb Company (BMY) engages in the discovery, development, license, manufacture, marketing, distribution, and sale of pharmaceutical and other health care products. Selected financial information for the company is as follows: TA 1 Year 2 Year 1 Revenue Net Income Total assets Total liabilities $19.4 billion 2.4 billion 30.4 billion 20.2 billion $20.9 billion 3.1 billion 27.5 billion 17.7 billion BMY's Year 2 annual report disclosed the following information in its footnote: Legal Proceedings and Contingencies Various lawsuits, claims, proceedings, and investiga- tions are pending against the Company and certain of its subsidiaries. In accordance with SFAS No. 5, Accounting for Contingencies, the Company records accruals for such contingencies when it is probable that a liability will be incurred and the amount of loss can be reasonably esti- mated. These matters involve antitrust, securities, patent infringements, the Employee Retire- ment Income Security Act of 1974, as amended (ERISA), pricing, sales and marketing practices. environmental health and safety matters, product liability and insurance coverage. The most significant of these matters are described on the next page. Plavix Litigation The Company's U.S. territory partnership under its alliance with Sanofi is a plaintiff in three pending patent infringement lawsuits instituted in the U.S. District Court for the Southern District of New York. Plaintiffs infringement position is based on defendants filing of their Abbreviated New Drug Application (ANDA) with the FDA, seeking approval to sell generic clopido- grel bisulfate prior to the expiration of the composition of matter patent in Year 9. Plavix is currently the Company's largest product ranked by net sales. Net sales of Plavix were approximately $3.3 billion and $2.5 billion for the years ended December 31, Year 2 and Year 1, respectively. Loss of market exclusivity of Plavix and the subsequent development of generic competition would be material to the Company's sales of Plavix and results of operations and cash flows and could be material to its financial condition and liquidity Legal Proceedings and Contingencies Various lawsuits, claims, proceedings, and investiga- tions are pending against the Company and certain of its subsidiaries. In accordance with SFAS No. 5, Accounting for Contingencies, the Company records accruals for such contingencies when it is probable that a liability will be incurred and the amount of loss can be reasonably esti- mated. These matters involve antitrust, securities, patent infringements, the Employee Retire- ment Income Security Act of 1974, as amended (ERISA), pricing, sales and marketing practices, environmental health and safety matters, product liability and insurance coverage. The most significant of these matters are described on the next page. Plavix Litigation The Company's U.S. territory partnership under its alliance with Sanofi is a plaintiff in three pending patent infringement lawsuits instituted in the U.S. District Court for the Southern District of New York. Plaintiffs infringement position is based on defendants' filing of their Abbreviated New Drug Application (ANDA) with the FDA, seeking approval to sell generic clopido grel bisultate prior to the expiration of the composition of matter patent in Year 9. Plavix is currently the Company's largest product ranked by net salos. Net sales of Plavix were approximately $3.3 billion and $2.5 billion for the years ended December 31, Year 2 and Year 1, respectively. LOSS of market exclusivity of Plavix and the subsequent development of generic competition would be material to the Company's sales of Plavix and results of operations and cash flows and could be material to its financial condition and liquidity, Required 1. Historically, the loss of patent protection on a brand name pharmaceutical product has resulted in a 70 percent decline in product sales. Assuming that (A) BMY patent protection on Plavix is lost as of the beginning of Year 3. (b) revenue and earnings for Year 3 would, in the absence of the loss of patent protection, have equaled those of Year 2, and (c) the after-tax margin on Plavix is 60 percent, estimate BMY'S revenue and comings for Year 3. How material are these financial effects? 2 BMY'S Plavix patent is carried on its balance as an intangible asset valued at $120 million. In the event of patent protection loss, how should the company reflect this in its financial statements? 3. How should the capital market react to (a) the disclosure that a patent infringement suit involving Plavix had been filed against BMY and (b) the disclosure that the suit had been lost (won)? D1031