Question
P4.14 Dimethyl carbonate (DMC, C3H6O3) can be synthesized by a process called oxidative carbonylation of methanol: 2CH3OH + CO + 1/102 CHO3 + HO
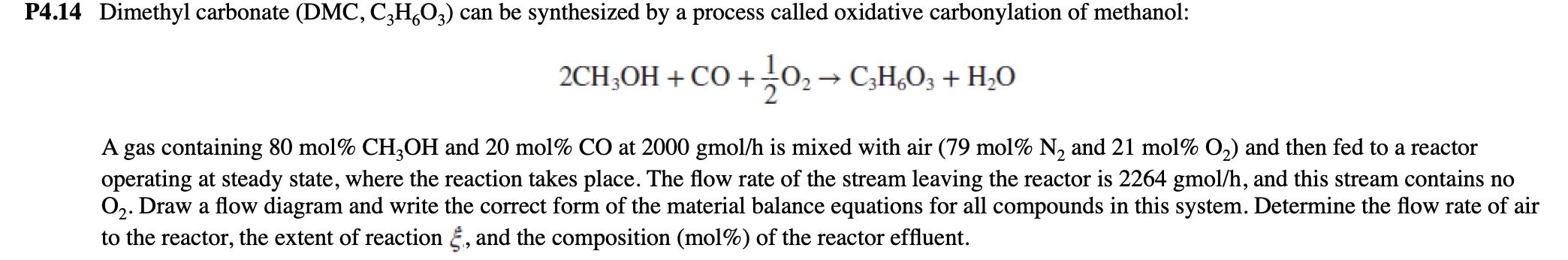
P4.14 Dimethyl carbonate (DMC, C3H6O3) can be synthesized by a process called oxidative carbonylation of methanol: 2CH3OH + CO + 1/102 CHO3 + HO A gas containing 80 mol% CH3OH and 20 mol% CO at 2000 gmol/h is mixed with air (79 mol% N2 and 21 mol% O2) and then fed to a reactor operating at steady state, where the reaction takes place. The flow rate of the stream leaving the reactor is 2264 gmol/h, and this stream contains no O2. Draw a flow diagram and write the correct form of the material balance equations for all compounds in this system. Determine the flow rate of air to the reactor, the extent of reaction &, and the composition (mol%) of the reactor effluent.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get StartedRecommended Textbook for
Organic Chemistry
Authors: Paula Yurkanis Bruice
4th edition
131407481, 978-0131407480
Students also viewed these Chemical Engineering questions
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
Question
Answered: 1 week ago
View Answer in SolutionInn App



