Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part 1: AHm dissolution Mass of Water Mass of NaOH Total Mass in cup Mole of NaOH Initial Temperature 21.0C Final Temperature 29.4C Change
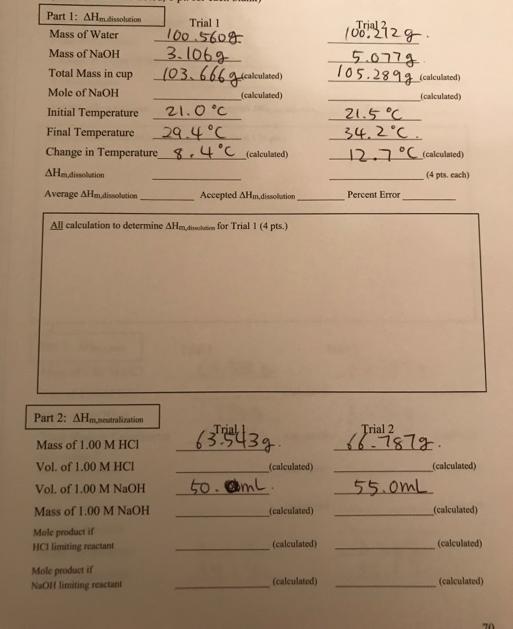
Part 1: AHm dissolution Mass of Water Mass of NaOH Total Mass in cup Mole of NaOH Initial Temperature 21.0C Final Temperature 29.4C Change in Temperature 8.4C (calculated) AHm dissolution Average AHm dissolution Part 2: AHmneutralization Mass of 1.00 M HCI Vol. of 1.00 M HCI Vol. of 1.00 M NaOH Mass of 1.00 M NaOH Mole product if HCI limiting reactant Trial 1. 100.5608 3.1069 103.6669 (calculated) (calculated) All calculation to determine AHm,d for Trial 1 (4 pts.) Mole product if NaOlf limiting reactant Accepted AHm dissolution 634 439. 50.@ml (calculated) (calculated) (calculated) (calculated) Trial 5.077g. 105.2899 (calculated) (calculated) 21.5C 34.2C. 12.7 C (calculated) (4 pts. each) Percent Error Trial 2 66.7872. 55.0mL (calculated) (calculated) (calculated) (calculated) 70
Step by Step Solution
★★★★★
3.56 Rating (167 Votes )
There are 3 Steps involved in it
Step: 1
The image provided appears to be a worksheet with data collected from experiments designed to measure enthalpy changes during a chemical reaction The ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


