Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part 1. Density of water 1. Volume of water. 2. Mass of beaker: 3. Mass of beaker + water: 4. Mass of water: 5.


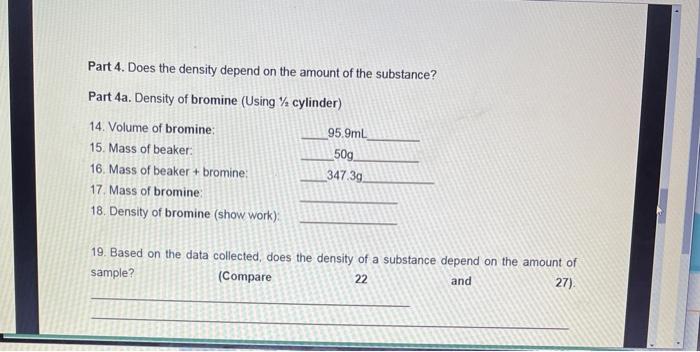
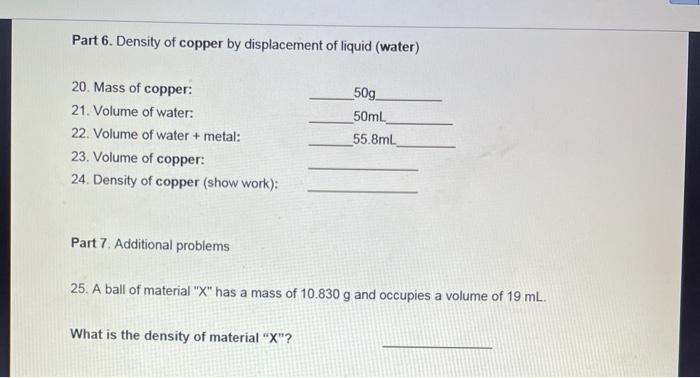

Part 1. Density of water 1. Volume of water. 2. Mass of beaker: 3. Mass of beaker + water: 4. Mass of water: 5. Density of water (show work): Part 2. Density of gasoline 6. Volume of gasoline: 7. Mass of beaker: 8. Mass of beaker + gasoline: 9. Mass of gasoline: 10. Density of gasoline (show work). 50mL 50g 100g 85mL 50g 116.3g Part 3. Miscibility of liquids Miscible liquids completely combine to form one solution. Immiscible liquids do not mix completely (heterogeneous mixture) Fill a graduated cylinder with " water and gasoline" 11. Are 12. Which water and gasoline miscible or immiscible? liquid is at the top? Which liquid is at the bottom? 13. Is this expected from your previously collected data? Explain in terms of density. (Compare 5 and 10) Part 4. Does the density depend on the amount of the substance? Part 4a. Density of bromine (Using cylinder) 14. Volume of bromine: 15. Mass of beaker: 16. Mass of beaker + bromine: 17. Mass of bromine: 18. Density of bromine (show work): 95.9mL 50g 347.3g 19. Based on the data collected, does the density of a substance depend on the amount of sample? (Compare 22 and 27). Part 6. Density of copper by displacement of liquid (water) 20. Mass of copper: 21. Volume of water: 22. Volume of water + metal: 23. Volume of copper: 24. Density of copper (show work): Part 7. Additional problems 50g 50ml 55.8mL 25. A ball of material "X" has a mass of 10.830 g and occupies a volume of 19 mL. What is the density of material "X"? 26. What will happen to a ball of material "X" if placed in a container with gasoline? Will the ball of material "X" float or sink? Explain in terms of density.
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


