

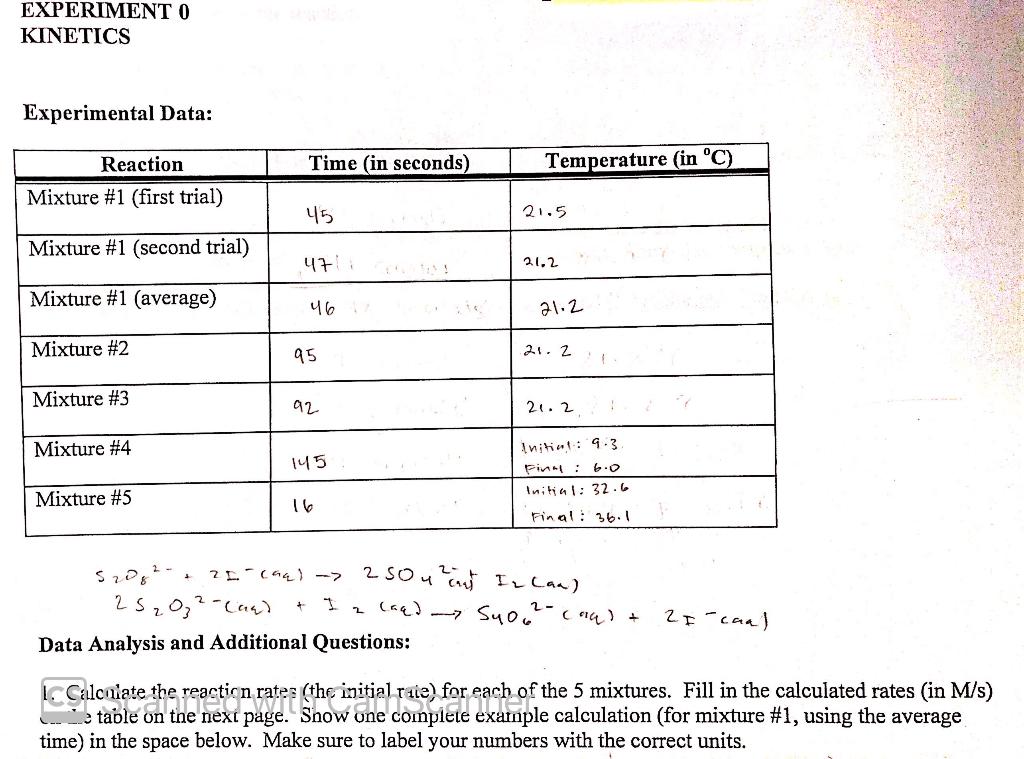
Part 1 - How the Concentration of the Reactants Affects the Reaction Rate. Rinse the pipet thoroughly with distilled water, then do a final rinse with a little of the 0.0050 M Na2S2O3 solution. Rinse both burets thoroughly with distilled water. Do a final rinse of one of the burets with 0.100 M(NH4)2S2Os, then fill that buret with 100 M (NH4)2S2O3. Do a final rinse of the other buret with 0,200 M KI, then fill that buret with 0.200 M KI. The other glassware should be clean, and any excess water shaken out. Label one of the Erlenmeyer flasks A and the other B. Mixture 1. Using your burets, measure 20.0 mL of 0.100 M (NH4)2S2Og into flask A and 20.0 mL of 0.200 MKI into flask B. Using the pipet, carefully measure 10.00 mL of 0.0050 M Na2S20; into the 250 mL beaker (this is the most critical measurement of the experiment). Add 3 drops of starch indicator to the beaker. Have your stopwatch ready to go, then quickly and simultaneously pour the contents of flasks A and B into the beaker and start the stopwatch. Swirl the beaker gently to promote mixing. Watch the reaction solution and stop the watch as soon as you see a blue color. Write down the time in seconds, to the nearest one second. Measure and record the temperature of the reaction solution. To get ready for the next trial, rinse the beaker with water and shake out the excess. The Erlenmeyer flasks don't need to be washed in between trails, but be sure to always use flask A for the persulfate and flask B for the iodide. Repeat the experiment for mixture #1. Your times for the two trials should be within a few seconds of each other. Mixture 2. The procedure is the same as for mixture 1, except that in flask A you will place 10.0 mL of 0.100 M(NH4)2S2O8 (measured from the buret) and 10.0 mL of 0.1 M (NH4)2SO4 (measured using a clean graduated cylinder), instead of 20,0 mL of 0.100 M (NH4)2S2O8. This will mean that the reaction mixture will have half the initial concentration of persulfate ion, but everything else will be the same. The (NH4)2SO4 is inert in this reaction- we use it instead of water to keep the total ionic composition of mixture 2 as close as possible to mixture 1. Record the time and temperature on your data sheet. Mixture 3. The procedure is the same as for mixture 1, except that in flask B you will place 10.0 mL of 0.200 M KI (measured from the buret) and 10.0 mL of 0.2 M KCI (measured using a clean graduated cylinder), instead of 20.0 mL of 0.200 M KI. This will mean that the reaction mixture will have half the initial concentration of iodide ion compared to mixture 1, but everything else will be the same. The KCl is inert in this reaction- we use it instead of water to keep the total ionic composition of mixture 3 as close as possible to mixture 1. Record the time and temperature on your data sheet. Part 2- How the Temperature Affects the Reaction Rate. Mixture 4. Mixture 4 is the same composition and procedure as mixture 1, but you will do the reaction at a temperature about 10 degrees below room temperature. To cool down the solutions, make a cold water bath by mixing some ice and water in a plastic tub. Check the temperature of the water bath and add enough ice to get the temperature of the water in tub somewhere between 5 and 10 C. After you dispense the reagents into the separate flasks and beaker, place them all in the cold water bath for 5 minutes to pre-chill them before you mix the solutions together. Once you mix the solutions together, leave the reaction beaker in the cold water bath for the duration of the reaction. Watch the reaction solution and stop the watch as soon as you see a blue color. Write down the time in seconds (be patient-it will take a long time at this temperature). Measure and record the temperature of the reaction solution. CS Scanned with CamScanner Mixture 5. Mixture 5 is the same composition and procedure as mixture 1, but you will do the reaction at a temperature about 10 degrees above room temperature. To warm up the solutions, make a warm water bath by using hot water from the tap, or by mixing tap water with water you have heated up in a large beaker using your Bunsen burner (Caution- handle the hot beaker with care!! You can make a potholder using a thick stack of folded paper towels.) Check the temperature of the water bath and adjust to get the temperature of the water in tub somewhere between 30 and 36C. After you dispense the reagents into the separate flasks and beaker, place them all in the warm water bath for 5 minutes to preheat them before you mix the solutions together. Once you mix the solutions together, leave the reaction beaker in the warm water bath for the duration of the reaction. Watch the reaction solution and stop the watch as soon as you see a blue color. Write down the time in seconds (be alert-it will react quickly at this temperature). Measure and record the temperature of the reaction solution. Data Analysis and Calculations. Calculating the Reaction Rate. Remember, the starch turns blue when all the thiosulfate has been consumed. To calculate the reaction rate (the initial rate) for each trial: 1. Calculate the initial moles of thiosulfate (S20337). This is the amount consumed in reaction #2 in the measured time interval. 2. Based on the moles of thiosulfate consumed, use the stoichiometric relations from reactions #1 and #2 (on the first page) to figure out how many moles of persulfate (S208) are consumed in reaction #1 during the same time interval. This is the change in moles of persulfate during the reaction. 3. Since the rate of the reaction is defined as the negative of the change in molarity of persulfate per time, calculate the reaction rate by dividing the change in moles of persulfate by the volume of the reaction solution and dividing by the time (in seconds). The formula is: --[5,03] moles of so consumed t L of reaction solution-time rate - Note: In this calculation we are assuming that the rate stays essentially constant during the measured time. This is a reasonable assumption since we are only running the reaction for a short period of time, so the [S2022] and the [T] don't change very much compared to their initial values. Calculating the Activation Energy. The relationship between the rate constant, the temperature, and the activation energy is given by the Arrhenius equation: k = Ae -EXT Where k is the rate constant, E, is the activation energy, R is the ideal gas constant, T is the absolute temperature, and A is the Arrhenius constant (or frequency factor). If you take the natural log of both sides of this equation, you can derive the relationship Ink = In A+ -E. RT 9 which can be written as Ink = (4)+ + In A R If you examine this relationship you will see that it is a linear equation (y = mx + b), where y = In k, x=1/T, and the slope is equal to -E/R. So to experimentally determine the activation energy for a reaction, you run the reaction at different temperatures (al other facieze held constant) and then make a graph of In k as a function of 1/T. The slope of this line is -E /R. EXPERIMENT O KINETICS Experimental Data: Time (in seconds) Temperature (in C) Reaction Mixture #1 (first trial) 45 21.5 Mixture #1 (second trial) 47 21.2 Mixture #1 (average) 46 21.2 Mixture #2 95 21. 2 Mixture #3 92 20.2 Mixture #4 145 Initiat: 9.3 Pind: 6.0 Initial: 32.6 Mixture #5 16 Final: 36.1 5208 2 L. (44) --> 2 so urant In Lan) 25202 - Land I a caed - Suou (14) + Data Analysis and Additional Questions: + Zi-caal 1. Calcaiate the reaction rates (the initial riie) for each of the 5 mixtures. Fill in the calculated rates (in M/s) the next page. Show one example calculation (for mixture #1, using the average time) in the space below. Make sure to label your numbers with the correct units. Solo le cumplien els









