Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A A 33 gram sample of copper (Csp(Cu) = 0.385 J/g K) is placed in a constant-pressure calorimeter containing 100 g of liquid water

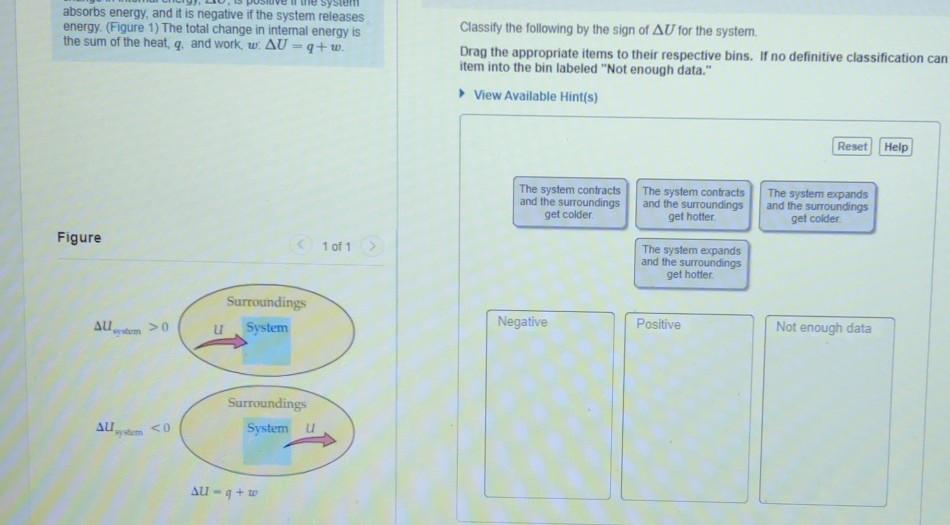

Part A A 33 gram sample of copper (Csp(Cu) = 0.385 J/g K) is placed in a constant-pressure calorimeter containing 100 g of liquid water at 99.5C right below the boiling temperature of 99.6C. After some time the temperature of the water becomes constant at 92.3C. What was the initial temperature of the copper block? Assume no heat is lost to the surroundings. absorbs energy, and it is negative if the system releases energy (Figure 1) The total change in internal energy is the sum of the heat, q, and work w. AU = 9+w. Classify the following by the sign of AU for the system, Drag the appropriate items to their respective bins. If no definitive classification can item into the bin labeled "Not enough data." View Available Hint(s) Reset Help The system contracts and the surroundings get colder The system contracts and the surroundings get hotter The system expands and the surroundings get colder Figure 1 of 1 The system expands and the surroundings get hotfer. Surroundings u System AU >0 Negative Positive Not enough data Surroundings System u AU,
Step by Step Solution
There are 3 Steps involved in it
Step: 1

Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


