Answered step by step
Verified Expert Solution
Question
1 Approved Answer
Part A A laboratory technician drops an 85.0 g solid sample of unknown material at a temperature of 100.0 C into a calorimeter. The
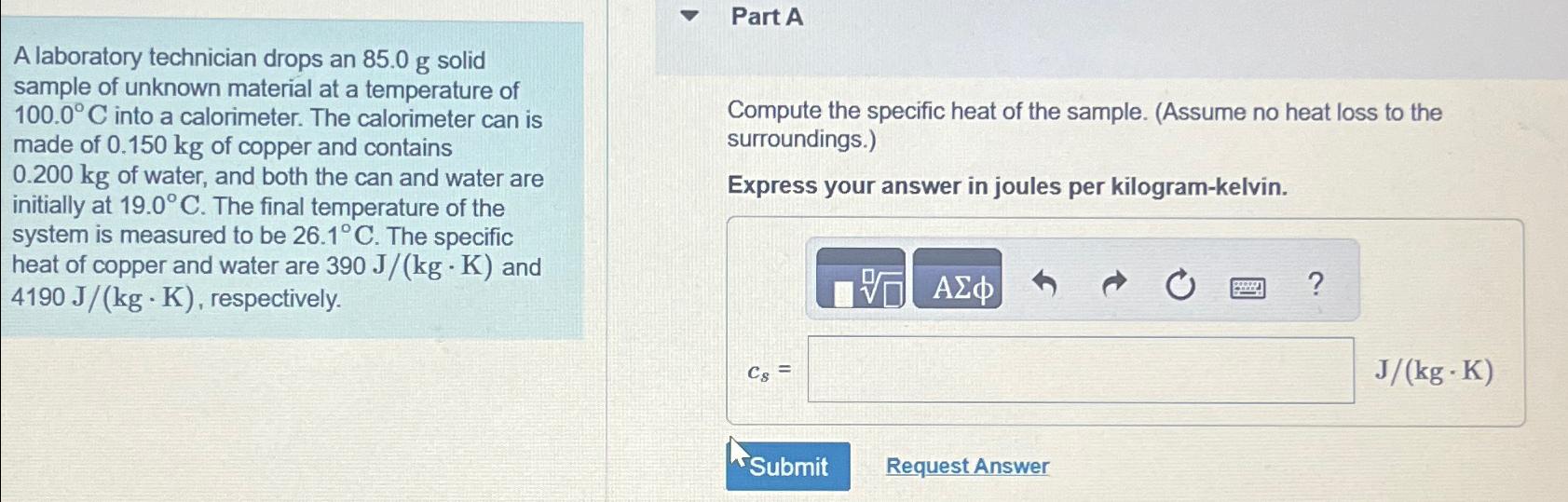
Part A A laboratory technician drops an 85.0 g solid sample of unknown material at a temperature of 100.0 C into a calorimeter. The calorimeter can is made of 0.150 kg of copper and contains 0.200 kg of water, and both the can and water are initially at 19.0 C. The final temperature of the system is measured to be 26.1C. The specific heat of copper and water are 390 J/(kg - K) and 4190 J/(kg K), respectively. Compute the specific heat of the sample. (Assume no heat loss to the surroundings.) Express your answer in joules per kilogram-kelvin. Cs= ? J/(kg -K) Submit Request Answer
Step by Step Solution
There are 3 Steps involved in it
Step: 1
To find the specific heat of the sample we can use the principle of conservation of energy The heat ...
Get Instant Access to Expert-Tailored Solutions
See step-by-step solutions with expert insights and AI powered tools for academic success
Step: 2

Step: 3

Ace Your Homework with AI
Get the answers you need in no time with our AI-driven, step-by-step assistance
Get Started


